Scheme 1.
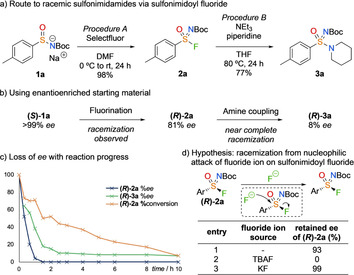
a) New route to sulfonimidamides via sulfonimidoyl fluorides was developed, however racemisation was observed in both fluorination and amine coupling steps, understood to be from sulfonimidoyl fluoride racemisation. For details of procedures A and B, see the Supporting Information. b) Loss of ee using enantioenriched materials under the initially developed conditions. c) ee and conversion with time for the reaction of enantioenriched (R)‐2 a and piperidine under procedure B. d) Reactions were performed on a 0.1 mmol scale in THF (0.3 m) at RT for 3 h. Retained ee given by % ee (R)‐2 a product/% ee (R)‐2 a SM (SM=starting material).
