Table 1.
Optimisation of amine coupling for retention of ee and yield.
|
| ||||||
|---|---|---|---|---|---|---|
|
Entry |
Solvent |
Additive |
Yield [%][a] |
[% es][c] |
||
|
|
|
|
(R)‐2 a |
(R)‐3 a |
total[b] |
|
|
1 |
THF |
– |
30 |
52 |
82 |
8 |
|
2 |
THF |
TMS‐Cl |
75 |
1 |
76 |
n.d. |
|
3 |
THF |
H2O |
6 |
77 |
83 |
26 |
|
4 |
THF |
KBr |
33 |
44 |
77 |
13 |
|
5 |
THF |
LiCl |
11 |
31 |
42 |
>99 |
|
6 |
THF |
LiBr |
19 |
56 |
75 |
>99 |
|
7 |
EtOH |
– |
– |
33 |
33 |
45 |
|
8 |
iPrOH |
– |
6 |
66 |
72 |
29 |
|
9 |
MeCN |
– |
9 |
73 |
82 |
28 |
|
10 |
MeCN |
LiBr |
– |
96 |
96 |
>99 |
|
11 |
MeCN |
LiI |
– |
87 |
87 |
>99 |
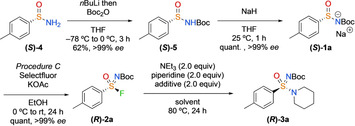
Optimisation of reaction conditions for yield and ee of (R)‐3 a. Reactions were performed using 0.1 mmol (R)‐2 a. [a] Yields determined by 1H NMR spectroscopy by using 1,3,5‐trimethoxybenzene as internal standard. [b] Sum of preceding two columns. [c] es=enantiospecificity, given by % ee (R)‐3 a/% ee (R)‐2 a. For details on Procedure C, see the Supporting Information.