Fig. 2.
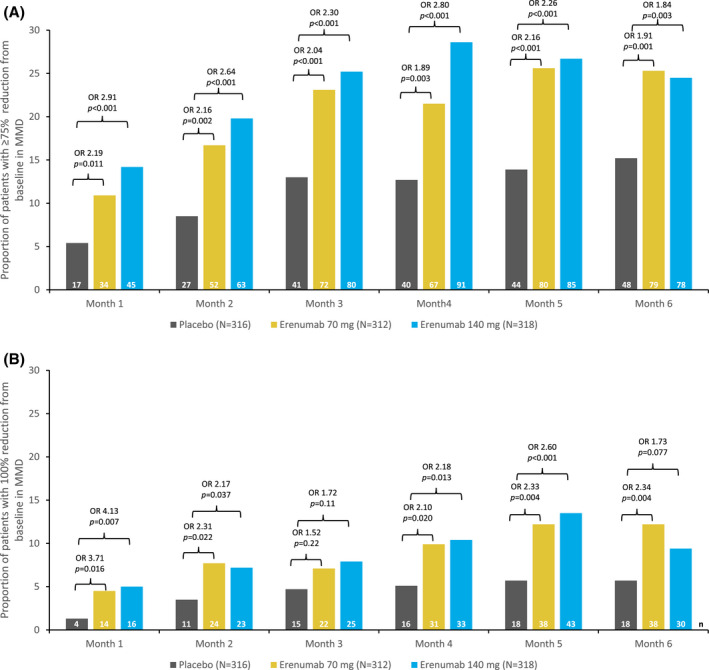
Proportion of patients with (A) ≥75% and (B) 100% reduction from baseline in monthly migraine days (MMD) during each month of the double‐blind treatment phase (DBTP). The common OR and P values are obtained from a Cochran‐Mantel‐Haenszel (CMH) test, stratified by stratification factors region and prior/current treatment with migraine prophylactic medication. The same analysis is repeated for each visit. The P values for pairwise comparisons are nominal P values obtained from the CMH test using data including placebo and corresponding erenumab dose group only. Proportion (%) = n/N * 100. (A) 95% confidence interval (CI) of the odds ratios (ORs) for month 1: 70 mg (1.19, 4.05) and 140 mg (1.62, 5.23); month 2: 70 mg (1.31, 3.55) and 140 mg (1.63, 4.27); month 3: 70 mg (1.33, 3.13) and 140 mg (1.51, 3.51); month 4: 70 mg (1.23, 2.92) and 140 mg (1.85, 4.25); month 5: 70 mg (1.43, 3.25) and 140 mg (1.51, 3.40); month 6: 70 mg (1.28, 2.85) and 140 mg (1.22, 2.76). (B) 95% CI of the ORs for month 1: 70 mg (1.20, 11.5) and 140 mg (1.36, 12.51); month 2: 70 mg (1.11, 4.81) and 140 mg (1.03, 4.53); month 3: 70 mg (0.77, 3.01) and 140 mg (0.88, 3.35); month 4: 70 mg (1.11, 3.95) and 140 mg (1.17, 4.07); month 5: 70 mg (1.29, 4.20) and 140 mg (1.46, 4.64); month 6: 70 mg (1.29, 4.23) and 140 mg (0.94, 3.21). Number in the efficacy analysis set (N); number in the subgroup (n).
