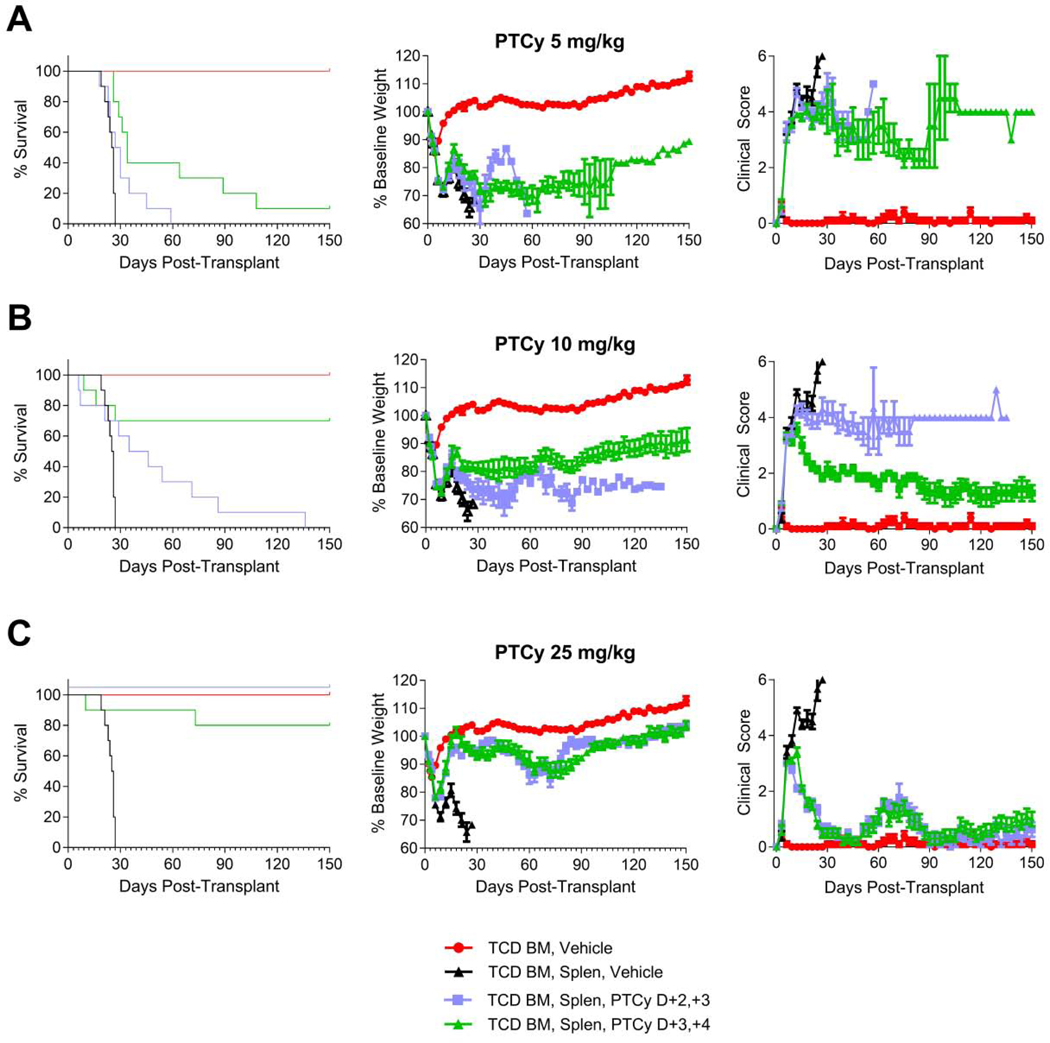
Figure 3. PTCy given on days +3/+4 is superior to days +2/+3 for suboptimal doses.
Mice were transplanted as in Figure 1 and were given PTCy on days +2/+3 or +3/+4 at (A) 5 mg/kg/day, (B) 10 mg/kg/day, or (C) 25 mg/kg/day. Mice were given PBS vehicle on days not receiving PTCy. The same TCD BM, Vehicle and TCD BM, Splen, Vehicle control groups are shown in all parts for comparison purposes. Statistical comparisons are between PTCy on days +2/+3 and on days +3/+4. (A) Survival was better for the 5 mg/kg/day PTCy dosing on days +3/+4, although the difference was not statistically significant (HR=2.7, p=0.061). Weight and clinical score AUCs were compared through day +21 and were not significantly different. (B) For the 10 mg/kg/day dosing, the survival of mice treated with PTCy on days +3/+4 was significantly superior compared with days +2/+3 (HR=5.6, p=0.0075). Although the weight AUCs through day +27 were not significantly different, the clinical score AUCs over that time interval were significantly better for 10 mg/kg/day PTCy on days +3/+4 (p=0.011). (C) There were no differences in survival or AUCs of weights or clinical scores between dosing schedules for mice receiving 25 mg/kg/day PTCy. For all parts, combined results are shown for two independent experiments of 5 mice/group/experiment.