Fig. 1.
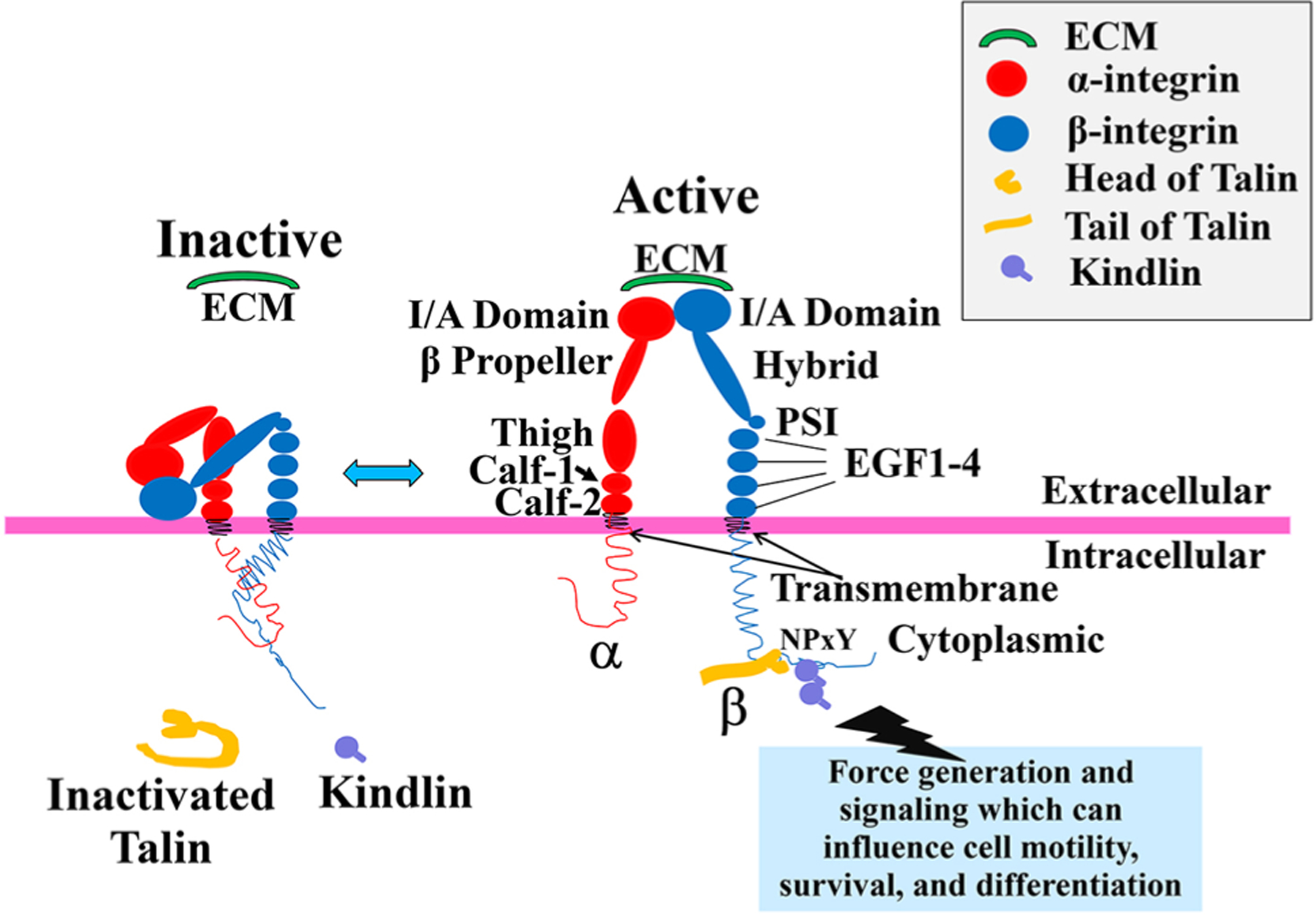
Integrin’s structure and its activation. Activation of integrin heterodimers leads to intracellular signaling (not shown) and results in processes such as cell motility, survival, and differentiation. Schematic shows integrin conformation at the membrane in the inactive and active conformations. In the inactive state, the extracellular domains of integrin heterodimers are present in a closed/bent conformation stabilized by a cytoplasmic salt bridge. This conformation has a very low ligand-binding affinity, e.g., for extracellular matrix (ECM). With initiation of “Inside–Out” signaling, a series of intracellular events occurs. This includes a structural change of talin from its autoinhibitory state, to an activated one as it unfolds. Then, the intracellular integrin activators, talin and kindlin, bind the β integrin cytoplasmic NPxY or Nxxy motif, respectively, leading to a conformational change of the integrin extracellular domain into an open and extended, i.e., active, state. In this state, the integrin I/A domain is exposed, and the integrin receptor has an increase in ligand-binding affinity for extracellular ligands such as the ECM proteins laminin, fibronectin, or collagen. As the extracellular ligands are bound, this initiates “Outside–In” signaling (not shown in this diagram) through the activated integrin, which initiates clustering of a series of intracellular proteins around the integrin cytoplasmic domains, and subsequent intracellular signaling events. In turn, this produces clustering of multiple integrin receptors, enabling a higher affinity and avidity for extracellular ligand, and thus teleologically, a firmer binding of integrins to ligands. Individual names of integrin extracellular domain components have been shown in the main text
