Graphical abstract
Keywords: HIV, COVID-19, SARS-CoV-2
Abstract
Objectives
Information on how COVID-19 affects people living with HIV (PLHIV) remains scarce.
Methods
An observational study was conducted in four public hospitals in Madrid. All HIV patients with confirmed or suspected COVID-19 were included and compared with COVID-19 patients without HIV infection.
Results
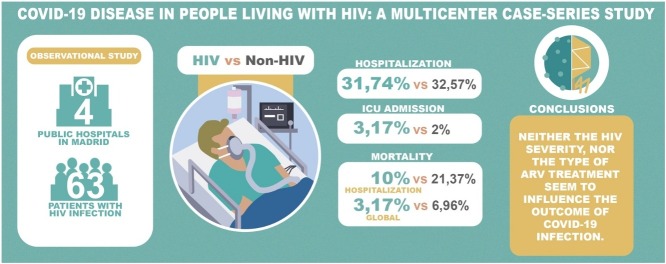
Sixty-three patients with HIV infection and confirmed or suspected COVID-19 were analyzed. The median age was 46 years (IQR: 37–56 years), and 88.9% were men. The median duration of HIV infection was 10.8 years (IQR: 6.5–16.8 years), and 96.8% were on antiretroviral therapy. 84.1% had previous comorbidities. The most common symptoms were fever (66.1%), cough (66.1%) and dyspnea (46.8%). Pneumonia was found in 47.5%, 28.6% of patients had severe disease, and 32.3% were admitted to hospital. The ICU admission rate and the mortality rate were both 3.17%. A significant association was observed between age, arterial hypertension, overweight, and diabetes mellitus and the severity of COVID-19. No association was observed between HIV-related factors and the severity of COVID-19. The rate of COVID-19 in HIV-patients was 1.68%. Similar hospitalization (31.74% vs 32.57%) and ICU admission (3.17% vs 2%) rates were observed with non-HIV infected patients. A lower mortality rate during hospitalization (10% vs 21.37%) and a lower global mortality rate (3.17% vs 6.96%) were also observed.
Conclusions
Established poor prognostic factors for COVID-19 patients, such as age and comorbidities, remain the main determinants for PLHIV. Neither the HIV severity nor the type of ARV treatment seem to influence the outcome of COVID-19. Large prospective cohorts are needed in order to establish the differences between HIV-positive and HIV-negative patients.
Introduction
On July 1, 2020 the SARS-CoV-2 pandemic had affected around 10 million people, causing over 510 000 deaths (Johns Hopkins University, 2020). On the other hand, more than 3.4 million patients had recovered, and many countries had slowly restarted their return to the new reality after the implementation of unprecedented non-pharmaceutical interventions, such as national lockdowns (Flaxman et al., 2020). Spain was one of the most affected countries, having reported its highest mortality in Madrid (Centro de Coordinación de Alertas y Emergencias Sanitarias and Sanidad, 2020), which had increased by 161% since 2019 (Centro Nacional de Epidemiología, 2020a). The main risk factors for fatality were older age, high blood pressure, overweight, and other concomitant conditions (Zhou et al., 2020).
Information on how COVID-19 infection affects people living with HIV (PLHIV) is still scarce. Several studies support the idea that PLHIV could have a lower risk of developing severe COVID-19 (Laurence, 2020, Ridgway et al., 2020, Doherty, 2020, Patel and Pella, 2020, Blanco et al., 2020), including a lower infection rate, possibly related to the observed in-vitro activity of various antiretroviral drugs, particularly protease inhibitors (Cao et al., 2020, Choy et al., 2020, Goldman et al., 2020, Elfiky, 2020, Chien et al., 2020), or maybe due to the characteristic immune dysregulation that occurs in PLHIV, which might avoid or limit the cytokine cascade response associated with severe or critical COVID-19 (Laurence, 2020).
This study aimed to describe the clinical presentation and incidence rate of COVID-19 in PLHIV, including patients treated in hospitals in Madrid.
Methods
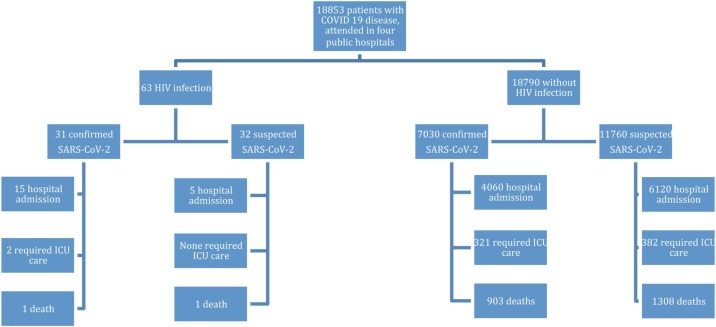
A retrospective observational study was conducted in the Quirónsalud network of public hospitals in the Community of Madrid, Spain: Fundación Jiménez Díaz University Hospital (HUFJD), Rey Juan Carlos University Hospital (HURJC), Infanta Elena University Hospital (HUIE), and Villalba General Hospital (HGV). From February 1 until May 20, 2020, 18 790 patients with confirmed or suspected COVID-19 disease were attended to. A total of 3738 HIV-infected patients are regularly followed-up in these four hospitals (HUFJD, 3373; HURJC, 130; HUIE, 144; HGV, 91). For our study, anonymized data were collected from electronic medical records. HIV-infected patients with probable or confirmed SARS-CoV-2 infection between February 1 and May 20 were included in the analysis.
A patient was classified as a probable case according to the Spanish Health Ministry protocol: clinical and radiological criteria of bilateral interstitial pneumonia consistent with COVID-19, or mild/acute respiratory infection along with epidemiological risk factors (relatives or close contacts with a proven diagnosis of COVID-19) (Centro de Coordinación de Alertas y Emergencias Sanitarias and Ministerio de Sanidad, 2020a). A case was defined as confirmed when the SARS-CoV-2 PCR was positive for a nasopharyngeal or throat swab. A waiver of consent was granted because only de-identified data were extracted from the medical records. Ethics committee approval was obtained from the institutional review board (EO 091/20). All research was performed according to the right to privacy, guaranteed as stipulated in EU Regulation 2016/679 of the European Parliament and of the Council of April 27, 2016 on General Data Protection Regulation (GDPR), regarding the protection of personal data, and the Declaration of Helsinki.
Data relating to demographics, baseline comorbidities, vital signs, peak laboratory tests, microbiological and imaging results, oxygen requirement, pharmacological treatments, and outcomes were obtained from all patients. Specific variables related to HIV were also collected: date of diagnosis, nadir and recent CD4 cell counts, years on antiretroviral therapy (ART) and virological suppression, and current antiretroviral regimen. The clinical picture associated with COVID-19 infection was classified according to the requirement for oxygen supply, as well as radiological criteria.
The study reference population data were obtained from the Community of Madrid and the national official register, both globally and by municipality (Anon, 2020a, Anon, 2020b). The following data were considered: a total of 6 663 394 people living in the Community of Madrid; 3 266 126 in the city of Madrid; 209 184 in Móstoles; 75 983 in Valdemoro; and 63 679 in Collado Villalba (Anon, a). The foreign-born population rates (per 100 000 inhabitants) were 14.13, 11.89, 9.53, and 17.16, respectively (Anon, 2020b, Anon, 2020c). By May 20, 2020, 66 860 cases of SARS-CoV-2 were diagnosed in the Community of Madrid; 42 442 required hospitalization, 3610 were admitted to the intensive care unit (ICU), and 8912 died (Centro de Coordinación de Alertas y Emergencias Sanitarias and Ministerio de Sanidad, 2020b, Madrid, 2020).
In order to avoid significant bias, our data were compared with the global data for patients attended to in the four hospitals, since patients who go to hospital might have not considered it possible to solve their symptoms by telephone assessment (Figure 1 ). Thus, both HIV-positive and HIV-negative patients had been treated with the same protocols in the same hospitals. Also, there was a previous report on the first 4712 consecutive hospitalized patients at these four centers (Heili-Frades et al., 2020).
Figure 1.
Patients flow chart.
Categorical variables were presented with frequencies and were compared using the Fisher’s exact test or Pearson’s chi-squared test. Continuous variables were presented as mean and standard deviation or median and interquartile range (IQR), and were compared using the Student’s t-test or the Mann–Whitney U test Logistic regression was used to test associations among variables and evaluate risk factors for COVID-19 in PLHIV. All statistical analyses were performed using SPSS 20, with a significance level of 0.05.
Results
Sixty-three patients with HIV infection who had SARS-CoV-2 infection were analyzed. The median age was 46 years (IQR 37–56 years) and 88.9% were male; 54% were Spanish and 36% Latin American. Regarding HIV infection, all but one (new HIV diagnosis) had been followed by their HIV specialist over the previous 3, 6, or 9 months. The median duration of HIV infection was 10.8 years (IQR 6.5–16.8 years), 96.8% were on antiretroviral therapy, 9.8% received protease inhibitor (PI)-based therapy, 63.9% were treated with integrase inhibitors, 26.2% with non-nucleoside reverse transcriptase analogs, and 14.8% with a tenofovir disoproxil fumarate-containing regimen. One patient was not on treatment due to personal choice. The median duration of continuous virological suppression (HIV-1 viral load < 50 cop/mL over the previous 6 months) was 7.2 years (IQR 3.3–12 years). 84.1% had previous comorbidities, with the most frequent being high blood pressure (HBP; 19%), cardiovascular events (12.7%), overweight (13%), and diabetes mellitus (DM; 9.5%). 48.2% were smokers or ex-smokers. The main clinical characteristics of HIV-infected patients are shown in Table 1 .
Table 1.
Epidemiological and HIV-related characteristics.
| N = 63 subjects | |
|---|---|
| Age (years)/median (IQR) | 46 (37–56) |
| Gender (male) | 88.9% |
| Country/region | |
| Spain | 54% |
| Latin-American | 36.5% |
| Europe | 6.3% |
| Others | 3.2% |
| HIV condition | |
| New diagnosis | 1 (1.6%) |
| Previous diagnosis | 62 (98.4%) |
| HIV infection time (years)/median (IQR) | 10.8 (6.5–16.8) |
| ART | 61 (96.8%) |
| PI-based therapy | 9.8% |
| INSTI-based therapy | 63.9% |
| NNRTIs-based therapy | 26.2% |
| TDF-containing regimen | 14.8% |
| TFV (TAF or TDF)-containing regimen | 26.2% |
| Virological suppression time (years)/median (IQR) | 7.2 (3.3–12) |
| CD4 (cel/mm3)/median (IQR) | 605 (391–921) |
| Nadir CD4 < 200 cel/mm3 | 26.7% |
| Comorbidities | |
| High blood pressure | 19% |
| Diabetes mellitus | 9.5% |
| Overweight | 13.1% |
| Cardiovascular disease | 12.7% |
| Chronic obstructive pulmonary disease | 4.8% |
| Renal chronic failure (CrCl < 30 mL/min) | 3.2% |
| Smoker | 48.2% |
| Current | 30.3% |
| Past | 17.9% |
The main characteristics relating to COVID-19 infection are shown in Table 2 . Almost half of the subjects (49.2%) had a firm diagnosis of COVID-19, either by PCR-SARS-CoV-2 or by serological tests. The other half (50.2%) were considered probable cases, according to the national protocol.
Table 2.
Main aspects related to COVID-19 disease.
| N = 63 patients | |
|---|---|
| COVID19 diagnosis | |
| Laboratory confirmed | 49.2% |
| PCR-SARS-CoV-2 | 38.1% |
| IgG-SARS-CoV-2 | 12.7% |
| IgM-SARS-CoV-2 | 6.3% |
| Suspected | 50.8% |
| Duration of symptoms (days)/median (IQR) | 6 (2–9.25) |
| Fever | 66.1% |
| Cough | 66.1% |
| Dyspnoea | 46.8% |
| Anosmia | 11.3% |
| Ageusia | 9.7% |
| Diarrhea | 22.6% |
| Headache | 14.5% |
| Weakness | 25.8% |
| Myalgia/arthralgia | 24.2% |
| Chest X-ray | 92.1% |
| Pneumonia | 47.5% |
| Unilateral | 32.1% |
| Bilateral | 67.9% |
| WHO Covid-19 severity score | 28.6% |
| CURB-65 score > 2 | 7.9% |
| Lymphopenia (< 1000/μL) | 26.5% |
| Ferritine > 1000 mcg/L | 25% |
| D-dimer > 2500 ng/mL | 4.8% |
| Hospital admission | 32.3% |
| Oxygen support requirements | 30.2% |
| FiO2 ≥ 0.5 | 6.7% |
| Chloroquine or Hydroxycloroquine | 49.2% |
| LMWH | 46% |
| Corticosteroids | 19% |
| Cyclosporine | 12.7% |
| Tocilizumab | 6.3% |
The median duration of symptoms before treatment was 6 days (IQR 2–9.25 days). The most prevalent symptoms were fever (66.1%), cough (66.1%), and dyspnea (46.8%). A chest X-ray was performed in 92.1% of patients. Pneumonia was observed in 47.5%; 67.9% of these suffered bilateral involvement (30.2% of the global cohort). According to the WHO COVID-19 severity score (World Health Organization, 2020), 28.6% of patients had severe disease. The CURB-65 score was ≥ 2 in five patients (7.9%), defined as critical disease.
Approximately one third of the cohort (32.3%) was admitted to hospital, with 30% of these requiring oxygen support (6.7% with FiO2 ≥ 0.5). In the overall sample, lymphopenia was present in 26.5%; 25% had ferritin levels over 1000 mcg/L and 4.8% had D-dimer values higher than 2500 ng/mL. Two patients required admission to the ICU (3.1%), and one of these died. One patient with a new HIV diagnosis with concomitant Pneumocystis jirovecii pneumonia (PCP) required admission to the ICU, with complete recovery after antimicrobial treatment for PCP and starting antiretroviral treatment. Over the following weeks, he developed SARS-CoV-2 infection with mild involvement and without pneumonia. Another patient died without ICU admission due to an advanced stage of an oncological disease.
All patients were treated according to the local and national protocols. Chloroquine or hydroxychloroquine (49.2%), low-molecular-weight heparine (46%), corticosteroids (19%), cyclosporine (12.7%), and tocilizumab (6.3%) were used according to standard therapy protocols.
In the univariate logistic regression analysis, a significant statistical association was observed between several risk factors, such as HBP, DM, overweight, or age, and the severity of COVID-19 (Table 3 ). No association was found with current CD4 value, a CD4 nadir < 200 cel/μL, or the regimen/type of antiretroviral therapy administered.
Table 3.
Potential risk factors related to severe disease.
| Severe disease N = 18 | Non-severe disease N = 45 | Logistic regression model(p-value) |
||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Age (years)/median (IQR) | 59 (52–70) | 41 (35–52) | <0.001 | 0.024 |
| HBP | 38.9% | 11.1% | 0.016 | 0.267 |
| DM | 22.2% | 4.4% | 0.048 | 0.814 |
| Overweight | 29.4% | 6.7% | 0.03 | 0.831 |
| Cardiovascular event | 27.8% | 6.7% | 0.034 | 0.932 |
| COPD | 16.7% | 0% | 0.999 | 1.000 |
| Tobacco | 83.3% | 38.6% | 0.013 | 0.785 |
| Current | 50% | 25% | 0.103 | 0.804 |
| Past | 33.3% | 13.6% | 0.126 | 0.848 |
| CD4 < 200 cel/μL | 16.7% | 2.2% | 0.068 | 0.355 |
| Nadir CD4 < 200 cel/μL | 33.3% | 22.2% | 0.363 | 0.825 |
| PI-containing regimen | 0% | 13.3% | 0.313 | 0.750 |
| TDF-containing regimen | 0% | 20% | 0.999 | 0.999 |
| TDF or TAF-containing regimen | 16.7% | 28.8% | 0.320 | 0.569 |
*HBP = high blood pressure; DM = diabetes mellitus; COPD = chronic obstructive pulmonary disease.
The global rate of COVID-19 infection in our HIV-patients cohort was between 0.86% (considering confirmed SARS-CoV-2 infections only) and 1.68% (considering both confirmed and suspected cases). By May 20, 2020 there were 66 860 confirmed COVID-19 patients in the Community of Madrid. Of these, 42 422 required hospitalization (63.47%) and 8912 died (13.32%; 21% of hospitalized patients). Over the same period, the rate of confirmed infections was 1% in the Community of Madrid, 1.1% in the city of Madrid, 1.02% in Móstoles, 1.05% in Valdemoro, and 0.83% in Collado Villalba (Anon, 2020b, Anon, 2020c). Differences between HIV-positive and HIV-negative patients, treated with the same protocols in the four hospitals, are described in Table 4 .
Table 4.
Comparison between HIV-infected patients and all patients with COVID-19 attending four public hospitals and living in the Community of Madrid.
| Patients with COVID19 attending four public hospitals |
Community of Madrid | ||
|---|---|---|---|
| HIV-infected patients | Non-HIV-infected patients | ||
| Confirmed | 31 | 7030 | 66 860 |
| Hospital admission (HA) | 15 (48.4%) | 4060 (57.7%) | 42 442 (63.5%) |
| H-admitted, died | 1 (6.7%) | 903 (22.2%) | 8912 (21%) |
| ICU care required | 2 (6.4%) | 321 (4.6%) | 3610 (5.4%) |
| HA ICU care required rate | 13.3% | 7.91% | 8.5% |
| Mortality rate | 3.2% | 12.8% | 13.3% |
| Confirmed and suspected | 63 | 18,790 | |
| HA | 20 (31.7%) | 6120 (32.6%) | |
| H-admitted, died | 2 (10%) | 1308 (21.4%) | |
| ICU care required | 2 (3.2%) | 382 (2%) | |
| HA ICU care required rate | 10% | 6.2% | |
| Mortality rate | 3.2% | 7% | |
Discussion
Our data show a COVID-19 infection rate in HIV-infected patients ranging from 0.86% to 1.68% — similar to those collected by the Community of Madrid and its municipalities (Anon, c). Regarding the general population, there was a lower hospitalization rate in confirmed cases (48.4% vs 63.47%). We also found a lower global mortality rate (3.22% vs 13.3%), including severe cases in patients admitted to hospital (6.67% vs 21%).
HIV-infected patients who had attended one of the four hospitals had a lower hospital admission rate and less mortality among both confirmed and confirmed/suspected patients when compared with non-HIV-infected patients. No differences were found in the rates of patients requiring ICU admission.
The SARS-CoV-2 infection rate in our HIV population was similar to that recently described in another large cohort in Madrid (Vizcarra et al., 2020). However, there were several differences found between our findings and those of Vizcarra et al. In spite of a similar severe disease rate (28.6% vs 25%), rates of both hospital admission (32.3% vs 55%) and patients requiring ICU care (3.1% vs 12%) were significantly lower than those found by Vizcarra et al. (2020) however, a similar mortality rate was observed (3.17% vs. 4%). Our data were closer to those of Gervasoni et al. (2020), with similar rates of hospital admission, severity, and ICU requirements.
These differences are probably driven by factors not related to HIV infection but to different general characteristics of the populations studied. Regarding Vizcarra et al. (2020), the mean age was slightly lower in our cohort, with lower rates of comorbidities; also, fewer patients had a history of severe immunodeficiency (< 200 CD4) and duration of HIV infection was shorter (10.8 years vs 19.5). Our data were even closer to those described by Gervasoni et al. (Gervasoni et al., 2020). Härter et al.’s cohort (Härter et al., 2020) had a similar mean age but showed higher severity, and higher rates of hospitalization, admission to ICU, and mortality. However, differences are more difficult to assess with a smaller sample and in the absence of data related to subject comorbidities; the same limitations were found when comparing with the Wuhan (Guo et al., 2020) and New York (Karmen-Tuohy et al., 2020, Suwanwongse and Shabarek, 2020) cohorts, both with a significantly higher median age. Finally, we found several similarities between our data and those of a recent systematic review (Mirzaei et al., 2020); in this case we suspect that the differences observed between the different cohorts are probably not significant.
Therefore, we consider that the severity of COVID-19 is related to the general characteristics of the population (age and comorbidities) and not to HIV infection, as we have observed in global cohorts (Richardson et al., 2020, Goyal et al., 2020), and in recent prospective (Inciarte et al., 2020, Etienne et al., 2020, Calza et al., 2020) and multicenter (Hadi et al., 2020) studies.
Another difference between the two Spanish cohorts lies in the prior antiretroviral treatment for HIV. The antiviral activity of lopinavir/ritonavir described against MERS or SARS (Park et al., 2019), as well as the recently demonstrated efficacy of remdesivir (Goldman et al., 2020) (a nucleotide analog that has similarities to tenofovir), have suggested that patients with HIV infection who are on antiretroviral therapy with protease inhibitors or tenofovir (TDF), may have a lower COVID-19 infection rate or severity (del Amo et al., 2020). No conclusions can be drawn with our data in this regard. Differences were observed with Vizcarra’s and Härter’s cohorts (Vizcarra et al., 2020, Härter et al., 2020), both with a higher prevalence of tenofovir in their patient regimens (73% and 66.7%, respectively). Our cohort had a lower prevalence of TDF-containing regimens (14.8%), closer to that of Gervasoni (42%) (Gervasoni et al., 2020). Probably due to the sample size, we could not find significant results in this regard. Nevertheless, several authors have described the potential activity of tenofovir agaist SARS-CoV-2 (Clososki et al., 2020, Salazar et al., 2020), but specific studies are required to answer this question.
The main limitation of our study was its retrospective condition, which entailed a lack of several data. Patients followed up in our clinic who were admitted for SARS-CoV-2 pneumonia at other facilities could not be included. In addition, data obtained from the Community of Madrid did not include some relevant characteristics, such as age, comorbidities, and other conditions, which could influence outcomes. Therefore, comparisons with our cohort were restricted. Our cohort included both probable and confirmed SARS-CoV-2 infections in the analysis due to the lack of resources for PCR testing in the first weeks of the pandemic in our region. Probable cases were considered by our four centers and other official protocols, despite not being confirmed by PCR. Our limited sample size, despite being the largest reported so far, did not allow more specific comparisons with the global non-HIV-infected COVID-19-infected population. Even though our HIV patients were younger than the general population treated in our hospitals (Heili-Frades et al., 2020), our general HIV patient cohort was broad enough to include patients from older groups, and yet those have not been primarily affected by SARS-CoV-2. In spite of these limitations, our data showed similarities to global national data published on the same date (Centro Nacional de Epidemiología, 2020b), which in our opinion supports the consistency of our findings.
Conclusions
Our study suggests that established poor prognostic factors for SARS-CoV-2 infection, such as older age and comorbidities, are also the main determinants for people living with HIV. Neither the severity of HIV infection, nor the scheme of HIV treatment or its efficacy seem to influence the outcome of COVID-19. Large prospective cohorts are needed in order to establish the differences between HIV-positive and HIV-negative patients.
Conflicts of interest
AC has received honoraria and speakers’ fees from Gilead Sciencies, MSD, Janssen-Cilag, and ViiV Healthcare. MG has received speakers’ fees from ViiV Healthcare and Gilead. BA reports personal fees from Gilead and ViiV Healthcare, outside the submitted work. All the authors have declared that no competing interests exist.
Funding
This work has not received funding. It has received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The author(s) received no specific funding for this work.
Ethical approval
The Medical Ethics Committee of the Fundación Jiménez Díaz University Hospital approved this study (reference approval number: EO091−020).
Authors’ contributions
AC, BZ, and MG conceived the study, participated in its design and data analysis, and drafted the manuscript. SN, VV, JH, LPP, IC, BA, RFR, MHS, JB, JMB, NR, RT, ALC, and AH participated in the study design and drafted the manuscript. All the authors contributed to the final version of the manuscript.
Acknowledgements
We would like to acknowledge Ignacio Mahillo for his collaboration in the statistical analysis and Drs Sánchez-Verde and Rodríguez de Lema for their contributions to graphical abstracts.
References
- Statistics National Institute.
- Statistical institute of the Community of Madrid.
- Comunidad de Madrid. No Title.
- Blanco J.L., Ambrosioni J., Garcia F. COVID-19 in patients with HIV: clinical case series. Lancet HIV. 2020;7(5):e314–e316. doi: 10.1016/S2352-3018(20)30111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza L., Bon I., Tadolini M. COVID-19 in patients with HIV-1 infection: a single-centre experience in northern Italy. Infection. 2020;(August) doi: 10.1007/s15010-020-01492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centro de Coordinación de Alertas y Emergencias Sanitarias, Ministerio de Sanidad . 2020. Procedimiento de actuación frente a casos de infección por le nuevo coronavirus (SARS-CoV-2). pp. 1–14. [Google Scholar]
- Centro de Coordinación de Alertas y Emergencias Sanitarias, Ministerio de Sanidad . 2020. Actualización no111. Enfermedad por el coronavirus (COVID19). 20.05.2020. Situación en España. [Google Scholar]
- Centro de Coordinación de Alertas y Emergencias Sanitarias, Sanidad Mde. 2020. Actualización No 132. Enfermedad Por El Coronavirus (COVID-19) 10.06.2020. [Google Scholar]
- Centro Nacional de Epidemiología . 2020. Vigilancia de los excesos de mortalidad por todas las causas: MoMo. Situación a 4 de junio de 2020; pp. 1–21. [Google Scholar]
- Centro Nacional de Epidemiología . 2020. Informe no32 sobre la situación de COVID-19 en España (21.05.2020) pp. 1–15. [Google Scholar]
- Chien M., Anderson T.K., Jockusch S. Nucleotide Analogues as Inhibitors of SARS-CoV-2 Polymerase. bioRxiv Prepr Serv Biol. 2020 doi: 10.1101/2020.03.18.997585. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy K.T., Wong A.Y.L., Kaewpreedee P. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178(April):104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clososki G., Soldi R., da Silva R. Tenofovir disoproxil fumarate: new chemical developments and encouraging in vitro biological results for SARS-CoV-2. J Braz Chem Soc. 2020;31(8):1552–1556. doi: 10.21577/0103-5053.20200106. [DOI] [Google Scholar]
- del Amo J., Polo R., Moreno S. Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy. Ann Intern Med. 2020;(25) doi: 10.7326/m20-3689. [DOI] [PubMed] [Google Scholar]
- Doherty M. COVID-19 and HIV latest WHO updates and guidance. Update 3 April 2020. World Heal Organ. 2020;(April) [Google Scholar]
- Elfiky A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253(February) doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne N., Karmochkine M., Slama L. HIV infection and COVID-19: risk factors for severe disease. Aids. 2020 doi: 10.1097/qad.0000000000002651. Publish Ah. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaxman S., Mishra S., Gandy A. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020 doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- Gervasoni C., Meraviglia P., Riva A. Clinical features and outcomes of HIV patients with coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa579. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J.D., Lye D.C.B., Hui D.S. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015301. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal P., Choi J.J., Pinheiro L.C. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Ming F., Dong Y. A survey for COVID-19 among HIV/AIDS patients in two districts of Wuhan, China. SSRN. 2020 [Google Scholar]
- Hadi Y.B., Naqvi S.F.Z., Kupec J.T., Sarwari A.R. Characteristics and outcomes of COVID-19 in patients with HIV: a multi-center research network study. AIDS. 2020 doi: 10.1097/QAD.0000000000002666. Publish Ah. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härter G., Spinner C.D., Roider J. COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection. 2020;(0123456789) doi: 10.1007/s15010-020-01438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heili-Frades S., Minguez P., Mahillo-Fernandez I. COVID-19 Outcomes in 4712 consecutively confirmed SARS-CoV2 cases in the city of Madrid. medRxiv. 2020 doi: 10.1101/2020.05.22.20109850. 2020.05.22.20109850. [DOI] [Google Scholar]
- Inciarte A., Gonzalez-Cordon A., Rojas J. Clinical characteristics, risk factors, and incidence of symptomatic COVID-19 in adults living with HIV. Aids. 2020 doi: 10.1097/qad.0000000000002643. Publish Ah. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins University . 2020. Coronavirus Resource Center. [Google Scholar]
- Karmen-Tuohy S., Carlucci P.M., Zervou F.N. Outcomes among HIV-positive patients hospitalized with COVID-19. J Acquir Immune Defic Syndr. 2020;53(9):1689–1699. doi: 10.1097/QAI.0000000000002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence J. Why aren’t people living with HIV at higher risk for developing severe coronavirus disease 2019 (COVID-19)? AIDS Patient Care STDS. 2020;34(6):1–2. doi: 10.1001/jama.2020.6548.8. [DOI] [PubMed] [Google Scholar]
- Madrid C.S.Cde. 2020. Datos COVID19 Comunidad de Madrid.https://www.comunidad.madrid/sites/default/files/doc/sanidad/200520_cam_covid19.pdf 843. [Google Scholar]
- Mirzaei H., McFarland W., Karamouzian M., Sharifi H. COVID-19 among people living with HIV: a systematic review. AIDS Behav. 2020 doi: 10.1007/s10461-020-02983-2. published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Lee J.S., Son J.S. Post-exposure prophylaxis for Middle East respiratory syndrome in healthcare workers. J Hosp Infect. 2019;101(1):42–46. doi: 10.1016/j.jhin.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel Rh, Pella Pm. COVID-19 in a patient with HIV infection. J Med Virol. 2020:0–3. doi: 10.1002/jmv.26049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA — J Am Med Assoc. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway J.P., Farley B., Benoit J.-L. A case series of five people living with HIV hospitalized with COVID-19 in Chicago, Illinois. AIDS Patient Care STDS. 2020 doi: 10.1089/apc.2020.0103. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar P.M.De, Ramos J., Cruz V.L., Polo R., Del Amo J., Mart J. Tenofovir and remdesivir ensemble docking with the SARS-CoV-2 polymerase and template-nascent RNA. authorea. 2020:1–8. doi: 10.22541/au.160133726.63184055. [DOI] [Google Scholar]
- Suwanwongse K., Shabarek N. Clinical features and outcome of HIV/SARS-CoV-2 coinfected patients in The Bronx, New York city. J Med Virol. 2020:0–1. doi: 10.1002/jmv.26077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcarra P., Pérez-Elías M.J., Quereda C. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;3018(20):1–11. doi: 10.1016/s2352-3018(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Clinical Management of COVID-19. WHO/2019-nCoV/clinical/2020.5. [Google Scholar]
- Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566–3. [DOI] [PMC free article] [PubMed] [Google Scholar]