Abstract
The uremic toxins indoxyl sulfate (IS) and p-cresyl sulfate (pCS) accumulate in patients with chronic kidney disease (CKD) as a consequence of altered gut microbiota metabolism and a decline in renal excretion. Despite of solid experimental evidence for nephrotoxic effects, the impact of uremic toxins on the progression of CKD has not been investigated in representative patient cohorts. In this analysis, IS and pCS serum concentrations were measured in 604 pediatric participants (mean eGFR of 27 ± 11 ml/min/1.73m2) at enrolment into the prospective Cardiovascular Comorbidity in Children with CKD study. Associations with progression of CKD were analyzed by Kaplan-Meier analyses and Cox proportional hazard models. During a median follow up time of 2.2 years (IQR 4.3–0.8 years), the composite renal survival endpoint, defined as 50% loss of eGFR, or eGFR <10ml/min/1.73m2 or start of renal replacement therapy, was reached by 360 patients (60%). Median survival time was shorter in patients with IS and pCS levels in the highest versus lowest quartile for both IS (1.5 years, 95%CI [1.1,2.0] versus 6.0 years, 95%CI [5.0,8.4]) and pCS (1.8 years, 95%CI [1.5,2.8] versus 4.4 years, 95%CI [3.4,6.0]). Multivariable Cox regression disclosed a significant association of IS, but not pCS, with renal survival, which was independent of other risk factors including baseline eGFR, proteinuria and blood pressure. In this exploratory analysis we provide the first data showing a significant association of IS, but not pCS serum concentrations with the progression of CKD in children, independent of other known risk factors. In the absence of comorbidities, which interfere with serum levels of uremic toxins, such as diabetes, obesity and metabolic syndrome, these results highlight the important role of uremic toxins and accentuate the unmet need of effective elimination strategies to lower the uremic toxin burden and abate progression of CKD.
Introduction
Identifying modifiable risk factors for the continuous loss of renal function that characterizes chronic kidney disease (CKD) is a major challenge towards improving treatment for patients with CKD. While hypertension and proteinuria are established risk factors for progression of CKD both in adults and children, even optimal current treatment still cannot prevent many patients progressing to end-stage kidney disease [1–3].
CKD leads to alterations in the gut microbiome (dysbiosis) and accumulation of gut derived uremic toxins in plasma [4]. We have previously shown that the uremic toxins indoxyl sulfate (IS) and p-cresyl sulfate (pCS) are inversely correlated to the estimated glomerular filtration rate (eGFR) in children and adolescents across all stages of CKD [5].
The accumulation of these toxins in the circulation has deleterious consequences, as their plasma concentrations are associated with cardiovascular events in adult CKD patients and with surrogate markers of cardiovascular disease (CVD) in pediatric patients [5, 6]. In addition, uremic toxins may affect kidney cells directly and thus promote progression of CKD. IS and pCS have direct toxic effects on tubular epithelial cells in vitro [7]. In animal models the accumulation of these toxins in renal tubular cells has been demonstrated to cause direct cytotoxicity, tubulointerstitial fibrosis and acceleration of CKD progression [8, 9]. While there is experimental evidence both in vitro and in vivo for nephrotoxic effects of uremic toxins, associations of IS and pCS serum levels with progression of CKD have only been investigated in a single study of adult CKD patients to date [10]. Here we investigated the impact of IS and pCS on progression of CKD in a cohort of more than 600 children with CKD of the prospective Cardiovascular Comorbidity in Children with Chronic Kidney Disease (4C) study.
Materials and methods
Study design
The 4C study is a prospective observational cohort study conducted at 55 pediatric nephrology centers in 12 European countries. Children with an initial eGFR of 10–60 ml/min per 1.73m2 were included in the study. Written informed consent was obtained from all patients and/or parents. The study was approved by the Ethics Board of the University of Heidelberg (S-032/2009) and subsequently by the local review boards of each participating institution (for a complete list of review boards, see [11]). All procedures performed involving human participants were in accordance with the Declaration of Helsinki. Additional details, including exclusion criteria, have been published in detail previously [12].
In the 4C study all participating centers were visited annually by trained regional coordinators. Every 6 months an update of the medical history and medication, clinical status and anthropometric data were obtained and blood and urine samples were collected and stored for central laboratory analysis. Office systolic blood pressure (SBP) was documented as an average of three oscillometric measurements using local devices. SBP and body mass index (BMI) were normalized for height and age (SBP SDS and BMI SDS respectively) as previously described. [12]. Glomerular filtration rate was estimated (eGFR) using the 2009 bedside Schwartz formula [13]. Patients were categorized according to their country of residency as Mediterranean (Turkey, Portugal, France and Italy) and non-Mediterranean (Germany, Austria, Switzerland, Poland, Lithuania, Serbia, Czech Republic and United Kingdom). Renal diagnoses were grouped into congenital anomalies of the kidney and urinary tract (CAKUT), tubulointerstitial disorders, glomerulopathies, chronic kidney disease after acute kidney injury (post-AKI CKD) and others.
Laboratory methods
Serum samples were available in 604 patients at the baseline visit. Total serum levels of IS and pCS were measured centrally using reverse-phase separation and fluorescence detection, as described previously [5].
Statistical analyses
Baseline characteristics are given as mean ± standard deviation (SD), median with interquartile range (IQR) or frequencies (n, %), as appropriate. Serum concentrations of IS and pCS measured at the baseline visit were used to analyze an association with renal survival time during follow up. Summary statistics stratified by CKD stage are provided for IS and pCS. As the distribution of IS and pCS was skewed, these variables were log transformed for further analysis. The assumption of normal distributed data was proven (visual inspection and comprehensive summary statistic) after log transformation. Correlation of IS and pCS (log transformed) with eGFR was quantified by means of Pearson correlation coefficient. In a multivariable linear regression analysis the association of log transformed IS (pCS) with clinically relevant variables at baseline was assessed [5].
The primary endpoint was renal survival defined as time from baseline to the composite event of either eGFR of <10ml/min or 50% loss in eGFR or start of RRT, whatever occurred first, as described previously [14, 15]; if eGFR<10 or 50% loss in eGFR occurred between two visits, linear interpolation was used to determine the “exact” point in time. As sensitivity analyses, two procedures were applied to prove consistency of our results because of not having observed the precise date of the event. First, instead of applying linear interpolation we used the later of the two visits as occurrence of the event. Second, subjects were interval-censored instead of linear interpolation. In the first case, analyses as described below can just be repeated as only the event time for some subjects changed. In the second case ICPHREG SAS procedure for interval-censored data was applied to the data with information on the two points between which the event occured. All data were administratively censored as of June 2019. Early drop-outs were censored at last contact and reasons for drop-out are summarized. Kaplan-Meier curves stratified by quartiles of IS or pCS levels are presented with p value of the log-rank test and median survival time. By means of a Cox proportional hazards model the association of IS or pCS with renal survival was analyzed providing adjusted hazard ratio (HR) with 95% confidence interval (CI) and p value. Different models were considered to evaluate if adding IS or pCS to the standard model (model 0) improved model fit. Covariates of model 0 were sex, renal diagnoses, residency, and baseline levels of eGFR, age, proteinuria (log transformed), SBP SDS, BMI SDS, serum albumin, hemoglobin and phosphorus. In model 1, IS or pCS was added to the set of basic covariates. By means of a forest plot the effect (HR with 95% CI) of IS (pCS) on renal survival was visualized for different patient characteristics (e.g. sex: male vs. female) based on separate Cox models with IS (pCS), the respective patient characteristic and the other remaining covariates included. Model 1 was repeated with categorized IS or pCS (quartile groups, compare Kaplan-Meier curves). Quality of the models was compared based on the Akaike information criterion (AIC), with lower values indicating a better fit. The AIC value is the expected, relative distance between the fitted model and the unknown true mechanism that generated the observed data.
A subset of patients with very high IS levels was identified by determining the upper 95% prediction limit (UPL) in a regression of eGFR on log-transformed IS (S1 Fig) 5. All analyses with respect to IS were carried out separately for the whole sample (total cohort) and, as sensitivity analyses, for the sample excluding patients with IS levels above the 95% UPL (consolidated cohort). As this was an exploratory study, p-values were interpreted descriptively and p<0.05 was considered statistically significant. Missing values were not imputed. Data were analyzed using SAS® Software version 9.4 (SAS Inc. Cary/ NC, USA).
Results
Patient characteristics
Between October 2009 and August 2011 a total of 704 children were enrolled in the 4C study. IS and pCS serum levels were measured in 604 patients with available blood samples and an eGFR between 10–60ml/min per 1.73 m2 at baseline denoted as total cohort in the following. Patient characteristics are given in Table 1; these are comparable to those in the entire 4C cohort [16]. Mean age was 12.1 ± 3.3 years with a mean eGFR of 27 ± 11 ml/min per 1.73 m2. CAKUT was the most common (70%) underlying primary renal disease.
Table 1. Patient characteristics.
| Age (years) | 12.1 ± 3.3 | n = 604 |
| Male sex | 399 (66%) | n = 604 |
| Diagnosis | n = 604 | |
| CAKUT | 420 (70%) | |
| Glomerulopathy | 49 (8%) | |
| Post-AKI | 27 (5%) | |
| Tubulointerstitial | 82 (14%) | |
| Others | 26 (4%) | |
| Country | n = 604 | |
| GER, CH, AT | 118 (20%) | |
| FR, IT, PT | 97 (16%) | |
| PL, LT, CZ, SRB | 53 (9%) | |
| UK | 35 (6%) | |
| Turkey | 301 (50%) | |
| BMI SDS | 0.08 ± 1.30 | n = 604 |
| Systolic blood pressure SDS | 0.81 ± 1.37 | n = 604 |
| eGFR (ml/min) | 26.6 ± 11.2 | n = 604 |
| uPCr (mg/mg) | 1.3 (2.6) | n = 579 |
| Serum Albumin (g/l) | 39.0 ± 6.30 | n = 604 |
| Serum phosphorus (mmol/l) | 1.55 ± 0.38 | n = 604 |
| Hemoglobin (g/l) | 11.6 ± 1.65 | n = 594 |
Data shown as mean ± standard deviation, median (interquartile range) or n (%) as appropriate. CAKUT = congenital anomalies of the kidney and urinary tract; Post-AKI = chronic kidney disease after acute kidney injury; BMI = body mass index; eGFR = estimated glomerular filtration rate; uPCr = Urinary protein creatinine ratio; SDS = standard deviation score. Countries: GER = Germany; CH = Switzerland; AT = Austria; FR = France; IT = Italy; PT = Portugal; PL = Poland; LT = Lithuania; CZ = Czech Republic; SRB = Serbia; UK = United Kingdom.
Measurement of indoxyl sulfate and p-cresyl sulfate
Serum IS and pCS concentrations were negatively correlated with eGFR (r = -0.46 for IS and r = -0.43 for pCS respectively). In 45 patients IS levels exceeded the 95% UPL calculated for the total cohort. These patients had a younger age (11.2 ± 3.3 versus 12.2 ± 3.3 years) and a lower hemoglobin (11.1 ± 1.70 versus 11.7 ± 1.64 g/l) compared to the rest of the cohort, but no other significant differences in baseline characteristics including medication with phosphate binders or antibiotics and iron supplementation (S1 Table). Additional sensitivity analyses were performed excluding these patients with IS levels exceeding the 95% UPL (denoted as consolidated cohort, S1 Fig and Table 2).
Table 2. Serum levels of indoxyl sulfate and p-cresyl sulfate according to CKD stage.
| Total cohort | CKD 3a | CKD 3b | CKD 4 | CKD 5 | Consolidated cohort | Excluded patients | |
|---|---|---|---|---|---|---|---|
| Patients (n) | 604 | 41 | 174 | 292 | 97 | 559 | 45 |
| IS mean (SD) | 25.5 (86.4) | 4.00 (11.7) | 17.8 (65.6) | 31.4 (105) | 30.8 (78.3) | 7.79 (9.95) | 246 (218) |
| IS median (IQR) | 5.30 (8.70) | 1.40 (1.60) | 3.45 (3.70) | 6.25 (7.30) | 13.0 (11.0) | 5.00 (6.80) | 178 (113) |
| IS quartiles | |||||||
| • <2.9 | 152 (25%) | 35 (85%) | 74 (43%) | 39 (13%) | 4 (4%) | 152 (27%) | 0 (0%) |
| • 2.9–5.3 | 152 (25%) | 4 (10%) | 58 (33%) | 85 (29%) | 5 (5%) | 152 (27%) | 0 (0%) |
| • 5.3–11.6 | 151 (25%) | 0 (0.0%) | 24 (14%) | 98 (33%) | 29 (30%) | 151 (27%) | 0 (0%) |
| • >11.6 | 149 (25%) | 2 (5%) | 18 (10%) | 70 (24%) | 59 (61%) | 104 (19%) | 45 (100%) |
| Patients (n) | 604 | 41 | 174 | 292 | 97 | 604 | 0 |
| pCS mean (SD) | 21.1 (18) | 6.99 (6) | 13.5 (11.2) | 22.4 (16.1) | 36.7 (22) | 21.1 (18) | |
| pCS median (IQR) | 17.2 (22) | 6.20 (7) | 9.80 (15.2) | 19.7 (19.7) | 34.1 (30) | 17.2 (22) | |
| pCS quartiles | |||||||
| • <7.9 | 153 (25%) | 26 (63%) | 67 (39%) | 49 (17%) | 11 (11%) | 153 (25%) | |
| • 7.9–17.2 | 149 (25%) | 13 (32%) | 53 (31%) | 74 (25%) | 9 (9%) | 149 (25%) | |
| • 17.2–29.6 | 151 (25%) | 1 (2%) | 41 (24%) | 92 (32%) | 17 (18%) | 151 (25%) | |
| • >29.6 | 151 (25%) | 1 (2%) | 13 (8%) | 77 (26%) | 60 (62%) | 151 (25%) |
Serum levels of indoxyl sulfate (IS) and p-cresyl sulfate (pCS) in the total cohort and across CKD stage 3–5 at study entry. For survival analyses, patients were grouped according to IS and pCS quartiles respectively. In the consolidated cohort, patients with IS levels > 95% of the upper prediction limit were excluded. IS and pCS serum levels in μmol/l. CKD = chronic kidney disease; IS = indoxyl sulfate; pCS = p-cresyl sulfate; SD = standard deviation; IQR = interquartile range.
In a multivariable regression analysis (S2 Table) IS levels showed positive associations with urea (p = 0.004) and negative associations with eGFR (p<0.001) and uric acid (p<0.001). Age, sex, diagnosis, residency, physical activity, BMI SDS, serum phosphorus, serum albumin, urinary protein/creatinine ratio and treatment with calcium-based phosphate binders, iron or antibiotics were not associated with IS serum levels. pCS levels were positively associated with age (p = 0.008), non-Mediterranean residency (p<0.001), serum albumin (p<0.001), urea (p = 0.038) and iron therapy (p = 0.034) and negatively associated with the diagnosis of glomerulopathies (p = 0.008), eGFR (p<0.001), uric acid (p = 0.002) and physical activity (> 4 hours per week, p = 0.044).
Progression of CKD
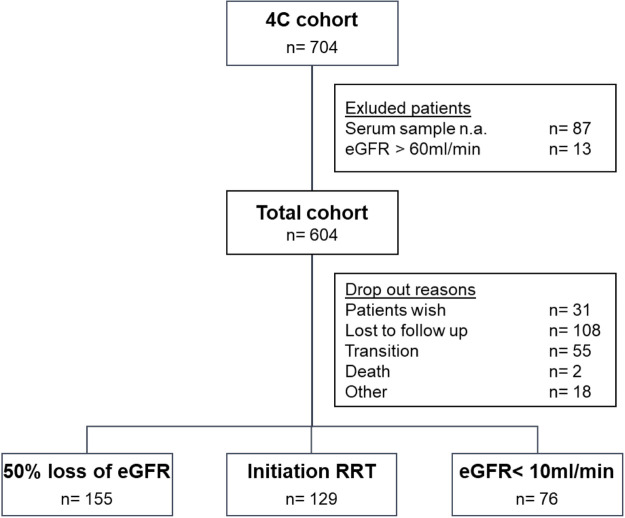
During a median follow up time of 2.2 years (IQR 4.3–0.8 years, maximum follow up 8.8 years), the composite renal survival endpoint was reached by 360 patients (59.6%). The median renal survival time was 3.5 years (95% CI, 2.9–3.9). Frequency and allocation of different endpoints as well as drop-out reasons are shown in Fig 1.
Fig 1. Study description with progression to primary endpoint and reasons for drop out.
eGFR = estimated glomerular filtration rate; RRT = renal replacement therapy.
Association of IS and pCS with progression of CKD
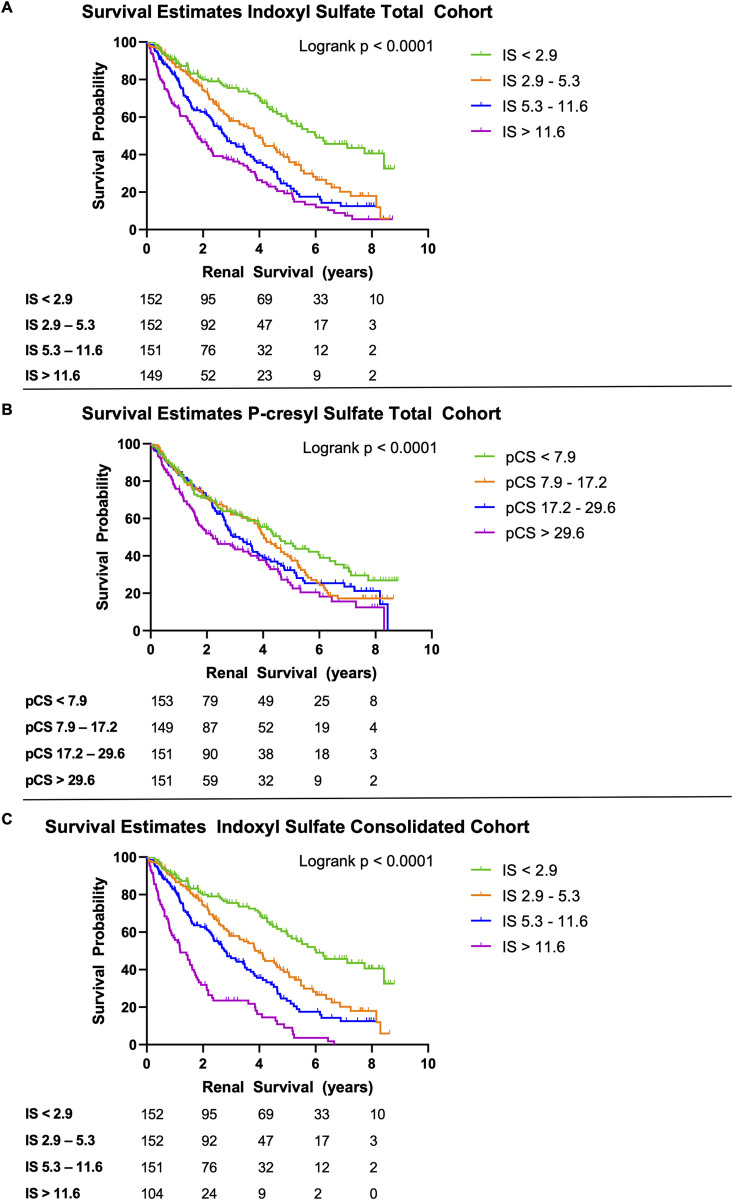
We found a significant association of serum IS and pCS concentrations with renal survival (p<0.0001, Fig 2A and 2B). Across all patients, the median survival time was lower in patients with IS and pCS levels in the 4th quartile (1.5 years, 95% CI [1.1, 2.0] for IS and 1.8 years, 95%CI [1.5, 2.8] for pCS) compared to those patients in the 1st quartile (6.0 years, 95% CI [5.0, 8.4] and 4.4 years, 95% CI [3.4, 6.0] for IS and pCS respectively). Exclusion of patients with IS levels above the 95% UPL (consolidated cohort), resulted in an even shorter median survival time (Fig 2C, 0.9 years, 95% CI [0.6, 1.5] of patients with IS levels in the 4th quartile).
Fig 2.
Renal survival in children with CKD according to quartiles of serum indoxyl sulfate (panels A, C) and p-cresyl sulfate (panel B). Results for total cohort (n = 604) are given in panels A and B. Panel C shows renal survival analysis by indoxyl sulfate quartiles after exclusion of patients with serum indoxyl sulfate above the 95% upper prediction limit (consolidated cohort, n = 559). Indoxyl sulfate and p-cresyl sulfate serum levels are given in μmol/l. Q1-4 indicate distribution quartiles.
In a multivariable Cox regression model, older age, male sex, diagnosis of tubulointerstitial disease or post-AKI CKD as well as the presence of proteinuria, higher systolic blood pressure, lower baseline eGFR, higher serum phosphorus, lower serum albumin and hemoglobin levels contributed to a higher likelihood of reaching the composite endpoint (Table 3 and Model 0, total cohort, Table 4). The levels of IS, but not pCS, contributed significantly to progression of CKD (Table 4). In detail, we found a significant association of IS levels in the 4th quartile compared to the 1st quartile and renal survival (Model 1b). Furthermore, after exclusion of patients with IS levels >95% UPL, IS as a continuous variable (Model 1e) was significantly associated with renal survival. The effect on renal survival of IS levels in the 4th quartile compared to the 1st quartile was even higher in patients of the consolidated cohort compared to patients of the total cohort (Model 1f, HR 1.94, 95% CI [1.29, 2.93] versus Model 1b, HR 1.62, 95% CI [1.12, 2.35]). Similar results were obtained for the additional sensitivity analyses without linear interpolation where the precise date of the event was not observed by taking the date of the later visit (S3 Table) or by considering interval censoring (S4 Table).
Table 3. Multivariable Cox regression model of variables associated with renal survival without indoxyl sulfate and p-cresyl sulfate (model 0, total cohort).
| Variable | Estimate | Standard Error | p-value | Hazard Ratio | 95% Hazard Ratio Confidence Intervall | |
|---|---|---|---|---|---|---|
| Female sex | -0.24 | 0.12 | 0.049 | 0.79 | 0.62 | 0.10 |
| Age (years) | 0.08 | 0.02 | < .001 | 1.09 | 1.05 | 1.13 |
| Diagnosis | ||||||
| Glomerulopathies | 0.24 | 0.22 | 0.261 | 1.28 | 0.83 | 1.96 |
| Post-AKI | 0.85 | 0.27 | 0.001 | 2.34 | 1.40 | 3.91 |
| Other | 0.18 | 0.30 | 0.558 | 1.19 | 0.66 | 2.16 |
| Tubulointerstitial | 0.74 | 0.16 | < .001 | 2.10 | 1.54 | 2.87 |
| Non-Mediterranean | 0.12 | 0.12 | 0.309 | 1.13 | 0.89 | 1.43 |
| BMI SDS | -0.02 | 0.05 | 0.682 | 0.98 | 0.90 | 1.07 |
| Systolic BP SDS | 0.15 | 0.04 | 0.001 | 1.17 | 1.08 | 1.26 |
| eGFR (ml/min/1.73m2) | -0.08 | 0.01 | < .001 | 0.93 | 0.91 | 0.94 |
| uPCr (mg/mg) | 0.34 | 0.06 | < .001 | 1.40 | 1.24 | 1.57 |
| Hemoglobin (g/l) | -0.14 | 0.04 | < .001 | 0.87 | 0.81 | 0.94 |
| Serum phosphorus (mmol/l) | 0.39 | 0.15 | 0.010 | 1.47 | 1.10 | 1.98 |
| Serum albumin (g/l) | -0.06 | 0.01 | < .001 | 0.94 | 0.92 | 0.96 |
Cox Model for the total cohort with variables measured at study entry (n = 569 patients). Diagnostic groups were compared to patients with the diagnosis of CAKUT. Non-Mediterranean residency was compared to patients living in Mediterranean countries. CAKUT = congenital anomalies of the kidney and urinary tract; Post-AKI = chronic kidney disease after acute kidney injury; BMI = body mass index; eGFR = estimated glomerular filtration rate; uPCr = Urinary protein creatinine ratio; SDS = standard deviation score.
Table 4. Multivariable Cox proportional hazard analysis for the association of IS and pCS serum levels with renal survival.
| AIC | Parameter | Estimate | Standard Error | p-value | Hazard Ratio | 95% Hazard Ratio Confidence Intervall | ||
|---|---|---|---|---|---|---|---|---|
| Model 0 total cohort | 3412.5 | --- | --- | --- | --- | |||
| Model 1a | 3412.2 | Log IS | 0.07 | 0.04 | 0.131 | 1.07 | 0.98 | 1.17 |
| Model 1b | 3400.3 | Q2: 2.9–5.3 | 0.15 | 0.18 | 0.426 | 1.16 | 0.80 | 1.65 |
| Q3: 5.3–11.6 | -0.15 | 0.19 | 0.422 | 0.86 | 0.59 | 1.25 | ||
| Q4: >11.6 | 0.48 | 0.19 | 0.011 | 1.62 | 1.12 | 2.35 | ||
| Model 1c | 3414.1 | Log pCS | -0.037 | 0.06 | 0.533 | 0.96 | 0.86 | 1.08 |
| Model 1d | 3416.7 | Q2: 7.9–17.2 | -0.22 | 0.17 | 0.201 | 0.80 | 0.57 | 1.12 |
| Q3: 17.2–29.6 | -0.17 | 0.18 | 0.317 | 0.84 | 0.60 | 1.18 | ||
| Q4: >29.6 | -0.13 | 0.18 | 0.464 | 0.88 | 0.61 | 1.25 | ||
| Model 0 consolidated cohort | 3088.5 | --- | --- | --- | --- | |||
| Model 1e | 3083.9 | Log IS | 0.20 | 0.08 | 0.001 | 1.23 | 1.05 | 1.43 |
| Model 1f | 3072.4 | Q2: 2.9–5.3 | 0.17 | 0.18 | 0.371 | 1.18 | 0.82 | 1.69 |
| Q3: 5.3–11.6 | -0.10 | 0.20 | 0.600 | 0.90 | 0.62 | 1.32 | ||
| Q4: >11.6 | 0.66 | 0.21 | 0.002 | 1.94 | 1.29 | 2.93 |
Model 0 represents a Cox model with sex, age, diagnosis, region of residence, body mass index SDS, blood pressure SDS, estimated glomerular filtration rate, urinary protein creatinine ratio, hemoglobin, serum phosphorus and albumin as covariates, calculated either for the total cohort (n = 569) or for the consolidated cohort (excluding patients with IS levels exceeding the 95% upper prediction limit, n = 526). Model 1a includes serum IS, 1b IS quartiles, 1c serum pCS and 1d serum pCS quartiles as a covariate to model 0. Models 1e and 1 f include serum IS and serum IS quartiles respectively as covariates to model 0 in the consolidated cohort. IS and pCS are used as logarithmic values in all models. For all variables associated with renal survival (model 0, total cohort) please refer to Table 3. IS = indoxyl sulfate; pCS = p-cresyl sulfate; Q = quartiles of serum IS and pCS levels, respectively; AIC = Akaike information criterion.
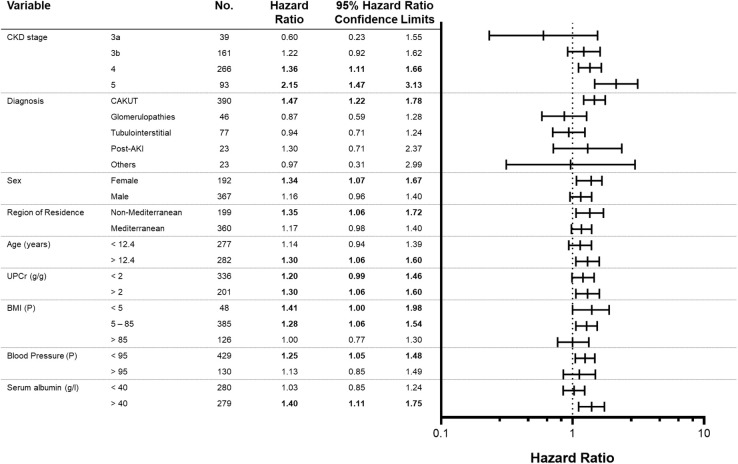
For the consolidated cohort, the effect of IS on renal survival in the different subsets adjusted for the other covariates is presented in Fig 3 by means of a forest plot. We found an interaction of IS levels with baseline CKD stage on renal survival, the highest hazard ratio being present in patients with CKD stage 5 at enrolment. Furthermore, the excess risk attributable to high IS concentrations was significantly elevated in normal-weight and normotensive, female and older patients, patients with CAKUT and those with non-Mediterranean residency.
Fig 3. Excess renal survival risk attributable to high versus low serum indoxyl sulfate levels according to patient characteristics of the consolidated cohort.
Each model was adjusted for all other covariates. Hazard ratio and 95% Confidence Limits indicate significance of interaction between IS status and respective patient characteristic. The median split of indoxyl sulfate was at 5.3 μmol/l. P = percentile; UPCr = urinary protein creatinine ratio.
Discussion
In this exploratory analysis we provide the first data showing the impact of microbial derived uremic toxins on the progression of CKD in a representative multicenter cohort of children with CKD. We found a significant association of serum IS concentrations with the prospective progression of CKD, independent of other known traditional risk factors, i.e. baseline eGFR, proteinuria and blood pressure. By contrast the pCS concentrations showed no association with renal survival after adjustment for baseline eGFR.
There is a growing body of evidence on how IS might contribute to the progression of CKD. In vitro, IS was shown to have direct toxic effects on tubular cells leading to increased levels of oxidative stress, fibrosis and inflammation [7, 17]. In animal models, IS seems to accelerate CKD progression by nephrotoxic effects [17]. Furthermore, vascular effects of IS might also contribute to progression of CKD. IS promotes vascular calcification [18] and inflammation [19] in vitro and in animal models of CKD [20]. Moreover, serum IS levels are associated with the surrogate markers of cardiovascular disease (intima media thickness and pulse walve velocity) in children [5] and with diminished endothelial function in adults with CKD [21]. Accordingly, the toxicity of IS in vitro and in animal models depends on its concentration, and this is reflected by the fact that those patients with IS levels in the 4th quartile have the highest risk for progression of CKD [22]. In the only clinical study in adult patients investigating the impact of IS and pCS serum levels on progression of CKD [10], 269 patients were followed up for 21 months; only 35 patients progressed to ESKD or had a decline in eGFR of 50% or more. In that group with progressive disease, there was a significant association of both IS and pCS serum concentrations with renal survival. However, almost half of the patients had diabetes, which is known to have a significant impact not only on the progression of CKD but also on the serum levels of uremic toxins [23]. Recently, it has been shown that lower kidney clearance of six endogenous solutes, including IS, but not pCS, are associated with a higher risk of CKD progression in adults participating in the CRIC study [24], which is in line with our results. In our study we provide the first results about the association of IS with progression of CKD in pediatric patients who had no other comorbidities which might interfere with renal survival. This allows to attribute the effect on renal survival more directly to serum concentrations of uremic toxins. This may explain why we identified a significant association with IS but not with pCS.
Our large study population permitted further statistical analysis of the relative contribution of IS levels to renal survival. The impact of IS levels increased with each stage of CKD, which is most likely due to accumulation of IS with progressive decrease of eGFR [5]. However, IS serum levels also showed interactions with other risk factors for progression. Thus, high IS levels were still associated with a moderately increased hazard ratio for renal progression in the presence of proteinuria, a diagnosis of CAKUT and for older patients. These are interesting findings as we have shown, that diagnosis of CAKUT itself had no direct influence on IS levels (S2 Table). Moreover, antibiotic treatment was more frequent in patients with CAKUT (29% of patients with CAKUT received antibiotic treatment or prophylaxis within six week before recruitment, S5 Table) compared to other patients (p<0.001), but antibiotics were not independently associated with IS levels. Thus the reason for the enhanced effect of IS in patients with CAKUT remains unexplained.
Proteinuria and low serum albumin are well known risk factors for progression of CKD, but neither proteinuria nor serum albumin were independently associated with IS levels. Nevertheless, IS was associated with a slightly higher hazard ratio for progression of CKD in patients with proteinuria, while the effect of IS on progression depending on serum albumin was decreased in hypoalbuminemia. Although we can not provide data about residual diuresis, measurement of free serum toxin concentrations or urinary toxins concentrations, these findings allow speculations about a higher fraction of free and therefore toxic serum concentrations of IS in patients with proteinuria and hypoalbuminemia. For female patients, our data show a lower hazard ratio than for male patients, i.e. better renal survival, but a significantly higher impact of higher serum levels of IS on renal survival.
Interestingly, the impact of higher IS levels on renal survival was stronger in patients with controlled blood pressure than in hypertensive patients; this finding could indicate that better blood pressure control during follow up, which occurred in this cohort (unpublished observation), led to “unmasking” of the effect of higher IS levels. Furthermore, a higher BMI-SDS was associated with better renal survival in the multivariable model, and the relative contribution of high IS levels to renal survival showed the highest hazard ratio in underweight, possibly malnourished patients. While obesity is regarded as an independent risk factor for progression of CKD in adults and children [25, 26], our data indicate the need for further studies regarding the role of uremic toxins in modulating progression of CKD in obese and non-obese patients. Taken together, our findings highlight the impact of elevated IS levels on renal survival and provide new insights into the pathophysiology of CKD progression in the absence of known risk factors such as obesity and arterial hypertension.
Therapeutic efforts to decelerate progression of CKD by lowering serum IS levels have so far been unsuccessful. A reduction of IS levels by administration of the oral adsorbent AST-120 had no significant effect on renal survival in clinical trials [27–29]. This might be explained by the complex interplay of nutrition and the gut microbiome, both of which essentially contribute to the balance of beneficial metabolites, e.g. short chain fatty acids (SCFAs), and harmful metabolites, e.g. uremic toxins, in CKD. Removal of uremic toxins by dialysis modalities, including intensified hemodialysis, is insufficient [30]. However, it has been shown that the administration of pre- and probiotics as well as SCFAs has beneficial effects on the gut microbiome and decreases the uremic toxin burden in vivo [31, 32]. Animal work has suggested that these effects of pre- and probiotics might also attenuate progression of CKD [33]. Therefore, studies using a combination of different treatment modalities such as microbiome-centered therapies (to restore a protective balance in microbiome composition and metabolism) and effective elimination strategies of uremic toxins are needed.
There are several limitations of our analysis. First, the 4C study was not designed specifically to evaluate the effect of microbial derived uremic toxins, but more general effects on progression of CKD. It is known that serum levels of gut-derived uremic toxins, such as IS and pCS, are potentially confounded by various factors such as age, diabetes, obesity and metabolic syndrome, which have a direct impact on the composition and function of the gut microbiome and hence, on microbial metabolism [34, 35]. Furthermore, antibiotic treatment is supposed to have an influence on serum levels of uremic toxins as well [31, 36]. Wherever possible, these factors have been addressed and included to our multivariable models. Nevertheless, it is unclear why some of our patients had extremely high serum levels of IS, but not pCS, above the 95% UPL. There were no significant differences between these patients and the rest of the cohort regarding baseline characteristics, including the frequency of antibiotic treatment or prophylaxis. We cannot completely rule out any pre-analytical bias in sampling, storage or transport to our central laboratory. However, the association of serum IS levels with renal survival was even stronger after exclusion of these patients. Furthermore, we have no information about symptomatic or asymptomatic gastrointestinal disorders. Nutrition and especially protein intake have an influence on microbial metabolism [37, 38] and have been associated with intra-patient variability, as described previously [39]. Although we have no data about nutrition and dietary intake in the 4C-study, we used Mediterranean residency as a surrogate parameter for nutrition. Furthermore, IS and pCS levels were measured only once at study entry, so we are not able to analyze the impact of nutrition on intra-patient variability in more detail.
Finally, we acknowledge the risk of attrition bias as a consequence of the high drop-out rate during our study follow up, limiting the power of our statistical analysis. On the other hand, the large number of patients enrolled in this study and the choice of a composite endpoint enables us to show a significant association of IS with progression of CKD for the first time in children.
In conclusion, our study shows a significant association of serum IS, but not pCS, with the progression of CKD in children, independent of other known risk factors such as baseline GFR, blood pressure and proteinuria. These results highlight the unmet need of effective elimination strategies to lower the uremic toxin burden, especially in patients with advanced CKD stages, in order to abate the progression of CKD.
Supporting information
Log-transformed serum levels of indoxyl sulfate (A) and p-cresyl sulfate (B) are correlated with eGFR in 604 children with chronic kidney disease at study entry. Linear regression of log-transformed serum levels of indoxyl sulfate (IS) and p-cresyl sulfate (pCS) showed a significant correlation with eGFR (Pearson correlation coefficient r = -0.46 for IS and r = -0.43 for pCS). 45 patients had IS levels above the 95% upper prediction limit (UPL). While no patients had pCS levels above the 95% UPL, some patients had pCS levels below the 95% lower prediction limit.
(PDF)
Data shown as mean ± standard deviation, median (interquartile range) or n (%) as appropriate. p-value indicates group difference between consolidated cohort and those patients with IS levels exceeding the 95% UPL. IS = indoxyl sulfate; UPL = upper prediction limit; CAKUT = congenital anomalies of the kidney and urinary tract; Post-AKI = chronic kidney disease after acute kidney injury; BMI = body mass index; eGFR = estimated glomerular filtration rate; uPCr = Urinary protein creatinine ratio; SDS = standard deviation score; Non-Ca phosphate binders = non calcium containing phosphate binders; Ca phosphate binders = calcium containing phosphate binders. Countries: GER = Germany; CH = Switzerland; AT = Austria; FR = France; IT = Italy; PT = Portugal; PL = Poland; LT = Lithuania; CZ = Czech Republic; SRB = Serbia; UK = United Kingdom.
(PDF)
Diagnostic groups were compared to patients with the diagnosis of CAKUT. Residency was defined as living in Mediterranean or Non-Mediterranean countries. Physical activity was compared to being physically inactive (0 hours). SE = standard error; CAKUT = congenital anomalies of the kidney and urinary tract; Post-AKI = chronic kidney disease after acute kidney injury; BMI = body mass index; eGFR = estimated glomerular filtration rate; Ca-based P binders = calcium-based phosphate binders; uPCr = Urinary protein creatinine ratio; SDS = standard deviation score.
(PDF)
Survival analysis using the PHREG SAS procedure. If the event occurred between two visits the later of the two visits was taken as occurrence of the event instead of using linear interpolation. Model 0 represents a Cox model with sex, age, diagnosis, region of residence, body mass index SDS, blood pressure SDS, estimated glomerular filtration rate, urinary protein creatinine ratio, hemoglobin, serum phosphorus and albumin as covariates, calculated either for the total cohort (n = 569) or for the consolidated cohort (excluding patients with IS levels exceeding the 95% upper prediction limit, n = 526). Model 1a includes serum IS, 1b IS quartiles, 1c serum pCS and 1d serum pCS quartiles as a covariate to model 0. Models 1e and 1 f include serum IS and serum IS quartiles respectively as covariates to model 0 in the consolidated cohort. IS and pCS are used as logarithmic values in all models. For all variables associated with renal survival (model 0, total cohort) please refer to Table 3. IS = indoxyl sulfate; pCS = p-cresyl sulfate; Q = quartiles of serum IS and pCS levels, respectively.
(PDF)
Survival analysis for interval-censored data using the ICPHREG SAS procedure. Model 0 represents a Cox model with sex, age, diagnosis, region of residence, body mass index SDS, blood pressure SDS, estimated glomerular filtration rate, urinary protein creatinine ratio, hemoglobin, serum phosphorus and albumin as covariates, calculated either for the total cohort (n = 569) or for the consolidated cohort (excluding patients with IS levels exceeding the 95% upper prediction limit, n = 526). Model 1a includes serum IS, 1b IS quartiles, 1c serum pCS and 1d serum pCS quartiles as a covariate to model 0. Models 1e and 1 f include serum IS and serum IS quartiles respectively as covariates to model 0 in the consolidated cohort. IS and pCS are used as logarithmic values in all models. For all variables associated with renal survival (model 0, total cohort) please refer to Table 3. IS = indoxyl sulfate; pCS = p-cresyl sulfate; Q = quartiles of serum IS and pCS levels, respectively.
(PDF)
CAKUT = congenital anomalies of the kidney and urinary tract.
(PDF)
Acknowledgments
The principal investigators of the 4C Study (4C Study consortium) included the following:
Pediatric Nephrology, University Children’s Hospital Vienna, Vienna, Austria: Klaus Arbeiter, MD; Department of Pediatrics I, Medical University, Innsbruck, Austria: Alejandra Rosales, MD; University Hospital Motol, Prague, Czech Republic: Jiri Dusek, MD; Pole Medico-Chirugical de Pediatrie, Hôpital de Hautepierre, Strasbourg, France: Ariane Zaloszyc, MD; Pediatric Nephrology, Charité Campus Virchow-Klinikum, Berlin, Germany: Uwe Querfeld, MD, and Jutta Gellermann, MD; Pediat-ric Nephrology Immunology and Hypertensiology, University Children’s Hospital, Cologne, Germany: Max Liebau, MD, and Lutz Weber, MD; Pediatric Nephrology, University Children’s Hospital, Erlan-gen, Germany: Evelin Muschiol, MD; Pediatric Nephrology, University Children’s Hospital, Essen, Germany: Rainer Büscher, MD; Pediatric Nephrology, UKE University Children’s Hospital, Hamburg, Germany: Jun Oh, MD; Pediatric Nephrology, Hannover Medical School, Hannover, Germany: An-ette Melk, MD, Daniela Thurn-Valassina, MD, and Dieter Haffner, MD; Division of Pediatric Nephrol-ogy, Center for Children and Adolescents, Heidelberg, Germany: Franz Schaefer, MD; Division of Pediatric Nephrology, Center for Pediatrics and Adolescent Medicine, Freiburg, Germany: Charlotte Gimpel, MD; Division of Pediatric and Nephrology, Center for Pediatrics and Adolescent, Jena, Germany: Ulrike John, MD; Children’s Dialysis Center, Town Hospital St Georg, Leipzig, Germany: Simone Wygoda MD; KfH Kidney Center for Children, Marburg, Germany: Nikola Jeck, MD; Pediatric Nephrology, University Children’s Hospital, Rostock, Germany: Marianne Wigger, MD; Pediatric Nephrology and Dialysis, Ospedale Maggiore, Policlinico, Milano, Italy: Sara Testa, MD; Dialysis and Transplantation, Department of Pediatrics, Dipartimento di Pediatria Salus Pueri, Padova, Italy: Luisa Murer, MD; Division di Nefrologia e Dialisi, Ospedale Pediatrico Bambino Gesú, Roma, Italy: Chiara Matteucci, MD; Center for Pediatrics, University Children’s Hospital, Vilnius, Lithuania: Augustina Jankauskiene, MD, and Karolis Azukaitis, MD; Dialysis Unit, PA Children’s Hospital, Cracow, Poland: Dorota Drozdz, MD; Pediatric Nephrology, Instituto Giannina Gaslini, Genova, Italy: Francesca Lugani, MD; Department of Pediatric and Adolescent Nephrology, University Medical School, Gdańsk, Poland: Aleksandra Zurowska, MD; Pomeranian Academy of Medicine, Clinic of Pediatrics, Szczecin, Poland: Marcin Zaniew, MD; Nephrology, Kidney, Transplantation and Hypertension, Children’s Memorial Health Institute, Warsaw, Poland: Mieczyslaw Litwin, MD, and Anna Nimierska, MD; Serviçio de Pediatria, Hospital de São João, Porto, Portugal: Ana Teixeira, MD; University Chil-dren’s Hospital, Belgrade, Serbia: Amira Peco-Antic, MD, and Dusan Paripovic, MD; Nephrology, University Children’s Hospital, Zürich, Switzerland: Guido Laube, MD; Cocuk Nefrolojisi Bilim Dali, Cukurova Universitesi Tip Fakültesi, Adana, Turkey: Ali Anarat, MD, and Aysun Bayazit, MD; Pediatric Nephrology, Hacettepe University, Ankara, Turkey: Ali Duzova, MD, and Yelda Bilginer, MD; Pediat-ric Nephrology, Cerrahpasa Medical Faculty, Istanbul, Turkey: Salim Caliskan, MD, Nur Canpolat, MD, and Mahmut Civilibal, MD; Pediatric Nephrology, Ege University, Izmir, Turkey: Sevgi Mir, MD, and Betül Sözeri, MD; Pediatric Nephrology, University Children’s Hospital, Münster, Germany: Brigitta Kranz, MD; Department of Pediatrics, Sant’Orsola-Malphighi Hospital, Bologna, Italy: Francesca Mencarelli, MD; Inselspital Children’s Hospital, Bern, Switzerland: Brigitte Dorn, MD; Pediatric Nephrology, University Tip Faculty of Medicine Cebeci Kampusu Cocuk, Ankara, Turkey: Fatos Yalcinkaya, MD; Faculty of Medicine, Baskent University, Ankara, Turkey: Esra Baskin, MD; Diskapi Children’s Hospital, Ankara, Turkey: Nilgun Cakar, MD; Pediatric Nephrology, Gazi University Hospital, Ankara, Turkey: Oguz Soylemezoglu, MD; Department of Pediatric Nephrology, Istanbul Medical Faculty, Çapa-Istanbul, Turkey: Sevinc Emre, MD; Pediatric Nephrology, Goztepe Educational and Research Hospital, Istanbul, Turkey: Cengiz Candan, MD; Pediatric Nephrology, Bakirkoy Children’s Hospital, Istanbul, Turkey: Aysel Kiyak, MD; Pediatric Nephrology, Sisli Educational and Research Hospital, Istanbul, Turkey: Gul Ozcelik, MD; Pediatric Nephrology, Marmara University Medical Faculty, Istan-bul, Turkey: Harika Alpay, MD; Nephrology, Great Ormond Street Hospital, London, United King-dom: Rukshana Shroff, MD; Service de Néphrologie Pédiatrique, Universite Hôpital Femme Mère Enfant, Lyon, France: Bruno Rachin, MD; Service de Pédiatrie, Centre de Reference Maladies Re-nales Rares du Sud-Quest, Bordeaux, France: Jerome Harambat, MD; Pediatric Nephrology, Zabrze, Poland: Maria Szczepanska, MD; Dortcelik Children’s Hospital, Bursa, Turkey: Hakan Erdogan, MD; Uludag University, Bursa, Turkey: Osman Donmez, MD; Department of Pediatric Nephrology, University of Gaziantep, Gaziantep, Turkey: Ayse Balat, MD; Tepecik Training and Research Hospital, Izmir, Turkey: Nejat Aksu, MD; Department of Pediatric Nephrology, Inonu Universtiy Medical School, Malatya, Turkey: Yilmaz Tabel, MD; Pediatric Nephrology Department, Celal Bayar University, Manisa, Turkey: Pelin Ertan, MD; and Pediatric Nephrology, Sanliurfa Children’s Hospital, Sanliurfa, Tur-key: Ebru Yilmaz, MD.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Support for this study was received from the European Renal Association - European Dialysis and Transplant Association, the KfH Foundation for Preventive Medicine, and the German Federal Ministry of Education and Research (reference number: 01EO0802). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Group ET, Wuhl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361(17):1639–50. Epub 2009/10/23. 10.1056/NEJMoa0902066 . [DOI] [PubMed] [Google Scholar]
- 2.Fathallah-Shaykh SA, Flynn JT, Pierce CB, Abraham AG, Blydt-Hansen TD, Massengill SF, et al. Progression of pediatric CKD of nonglomerular origin in the CKiD cohort. Clin J Am Soc Nephrol. 2015;10(4):571–7. Epub 2015/01/31. 10.2215/CJN.07480714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai WC, Wu HY, Peng YS, Ko MJ, Wu MS, Hung KY, et al. Risk Factors for Development and Progression of Chronic Kidney Disease: A Systematic Review and Exploratory Meta-Analysis. Medicine (Baltimore). 2016;95(11):e3013 Epub 2016/03/18. 10.1097/MD.0000000000003013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, et al. Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012;23(7):1258–70. Epub 2012/05/26. 10.1681/ASN.2011121175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holle J, Querfeld U, Kirchner M, Anninos A, Okun J, Thurn-Valsassina D, et al. Indoxyl sulfate associates with cardiovascular phenotype in children with chronic kidney disease. Pediatr Nephrol. 2019. Epub 2019/08/21. 10.1007/s00467-019-04331-6 . [DOI] [PubMed] [Google Scholar]
- 6.Velasquez MT, Centron P, Barrows I, Dwivedi R, Raj DS. Gut Microbiota and Cardiovascular Uremic Toxicities. Toxins (Basel). 2018;10(7). Epub 2018/07/13. 10.3390/toxins10070287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol. 2014;25(9):1897–907. Epub 2014/05/09. 10.1681/ASN.2013101062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takada T, Yamamoto T, Matsuo H, Tan JK, Ooyama K, Sakiyama M, et al. Identification of ABCG2 as an Exporter of Uremic Toxin Indoxyl Sulfate in Mice and as a Crucial Factor Influencing CKD Progression. Sci Rep. 2018;8(1):11147 Epub 2018/07/26. 10.1038/s41598-018-29208-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med. 1994;124(1):96–104. Epub 1994/07/01. . [PubMed] [Google Scholar]
- 10.Wu IW, Hsu KH, Lee CC, Sun CY, Hsu HJ, Tsai CJ, et al. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26(3):938–47. Epub 2010/10/05. 10.1093/ndt/gfq580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyon A, Fischer DC, Bayazit AK, Canpolat N, Duzova A, Sozeri B, et al. Markers of bone metabolism are affected by renal function and growth hormone therapy in children with chronic kidney disease. PLoS One. 2015;10(2):e0113482 Epub 2015/02/07. 10.1371/journal.pone.0113482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Querfeld U, Anarat A, Bayazit AK, Bakkaloglu AS, Bilginer Y, Caliskan S, et al. The Cardiovascular Comorbidity in Children with Chronic Kidney Disease (4C) study: objectives, design, and methodology. Clin J Am Soc Nephrol. 2010;5(9):1642–8. Epub 2010/06/26. 10.2215/CJN.08791209 CJN.08791209 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–37. Epub 2009/01/23. 10.1681/ASN.2008030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaefer F, Trachtman H, Wuhl E, Kirchner M, Hayek SS, Anarat A, et al. Association of Serum Soluble Urokinase Receptor Levels With Progression of Kidney Disease in Children. JAMA Pediatr. 2017;171(11):e172914 Epub 2017/09/06. 10.1001/jamapediatrics.2017.2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azukaitis K, Ju W, Kirchner M, Nair V, Smith M, Fang Z, et al. Low levels of urinary epidermal growth factor predict chronic kidney disease progression in children. Kidney Int. 2019;96(1):214–21. Epub 2019/04/22. 10.1016/j.kint.2019.01.035 . [DOI] [PubMed] [Google Scholar]
- 16.Schaefer F, Doyon A, Azukaitis K, Bayazit A, Canpolat N, Duzova A, et al. Cardiovascular Phenotypes in Children with CKD: The 4C Study. Clin J Am Soc Nephrol. 2017;12(1):19–28. Epub 2016/11/09. 10.2215/CJN.01090216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castillo-Rodriguez E, Fernandez-Prado R, Esteras R, Perez-Gomez MV, Gracia-Iguacel C, Fernandez-Fernandez B, et al. Impact of Altered Intestinal Microbiota on Chronic Kidney Disease Progression. Toxins (Basel). 2018;10(7). Epub 2018/07/22. 10.3390/toxins10070300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He X, Jiang H, Gao F, Liang S, Wei M, Chen L. Indoxyl sulfate-induced calcification of vascular smooth muscle cells via the PI3K/Akt/NF-kappaB signaling pathway. Microsc Res Tech. 2019;82(12):2000–6. Epub 2019/08/27. 10.1002/jemt.23369 . [DOI] [PubMed] [Google Scholar]
- 19.Nakano T, Katsuki S, Chen M, Decano JL, Halu A, Lee LH, et al. Uremic Toxin Indoxyl Sulfate Promotes Proinflammatory Macrophage Activation Via the Interplay of OATP2B1 and Dll4-Notch Signaling. Circulation. 2019;139(1):78–96. Epub 2018/12/28. 10.1161/CIRCULATIONAHA.118.034588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opdebeeck B, Maudsley S, Azmi A, De Mare A, De Leger W, Meijers B, et al. Indoxyl Sulfate and p-Cresyl Sulfate Promote Vascular Calcification and Associate with Glucose Intolerance. J Am Soc Nephrol. 2019;30(5):751–66. Epub 2019/04/04. 10.1681/ASN.2018060609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang CH, Lai YH, Kuo CH, Lin YL, Tsai JP, Hsu BG. Association between Serum Indoxyl Sulfate Levels and Endothelial Function in Non-Dialysis Chronic Kidney Disease. Toxins (Basel). 2019;11(10). Epub 2019/10/17. 10.3390/toxins11100589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu WC, Shyu JF, Lim PS, Fang TC, Lu CL, Zheng CM, et al. Concentration and Duration of Indoxyl Sulfate Exposure Affects Osteoclastogenesis by Regulating NFATc1 via Aryl Hydrocarbon Receptor. Int J Mol Sci. 2020;21(10). Epub 2020/05/21. 10.3390/ijms21103486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koppe L, Fouque D, Soulage CO. Metabolic Abnormalities in Diabetes and Kidney Disease: Role of Uremic Toxins. Curr Diab Rep. 2018;18(10):97 Epub 2018/09/09. 10.1007/s11892-018-1064-7 . [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Zelnick LR, Wang K, Hoofnagle AN, Becker JO, Hsu CY, et al. Kidney Clearance of Secretory Solutes Is Associated with Progression of CKD: The CRIC Study. J Am Soc Nephrol. 2020;31(4):817–27. Epub 2020/03/25. 10.1681/ASN.2019080811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun HR, Kim H, Park JT, Chang TI, Yoo TH, Kang SW, et al. Obesity, Metabolic Abnormality, and Progression of CKD. Am J Kidney Dis. 2018;72(3):400–10. Epub 2018/05/08. 10.1053/j.ajkd.2018.02.362 . [DOI] [PubMed] [Google Scholar]
- 26.Nehus E. Obesity and chronic kidney disease. Curr Opin Pediatr. 2018;30(2):241–6. Epub 2018/01/19. 10.1097/MOP.0000000000000586 . [DOI] [PubMed] [Google Scholar]
- 27.Liu WC, Tomino Y, Lu KC. Impacts of Indoxyl Sulfate and p-Cresol Sulfate on Chronic Kidney Disease and Mitigating Effects of AST-120. Toxins (Basel). 2018;10(9). Epub 2018/09/14. 10.3390/toxins10090367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulman G, Berl T, Beck GJ, Remuzzi G, Ritz E, Shimizu M, et al. Risk factors for progression of chronic kidney disease in the EPPIC trials and the effect of AST-120. Clin Exp Nephrol. 2018;22(2):299–308. Epub 2017/07/26. 10.1007/s10157-017-1447-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulman G, Berl T, Beck GJ, Remuzzi G, Ritz E, Shimizu M, et al. The effects of AST-120 on chronic kidney disease progression in the United States of America: a post hoc subgroup analysis of randomized controlled trials. BMC Nephrol. 2016;17(1):141 Epub 2016/10/08. 10.1186/s12882-016-0357-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snauwaert E, Van Biesen W, Raes A, Glorieux G, Vande Walle J, Roels S, et al. Haemodiafiltration does not lower protein-bound uraemic toxin levels compared with haemodialysis in a paediatric population. Nephrol Dial Transplant. 2019. Epub 2019/07/31. 10.1093/ndt/gfz132 . [DOI] [PubMed] [Google Scholar]
- 31.Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM, et al. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin J Am Soc Nephrol. 2016;11(2):223–31. Epub 2016/01/17. 10.2215/CJN.05240515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzocco S, Fazeli G, Di Micco L, Autore G, Adesso S, Dal Piaz F, et al. Supplementation of Short-Chain Fatty Acid, Sodium Propionate, in Patients on Maintenance Hemodialysis: Beneficial Effects on Inflammatory Parameters and Gut-Derived Uremic Toxins, A Pilot Study (PLAN Study). J Clin Med. 2018;7(10). Epub 2018/10/03. 10.3390/jcm7100315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaziri ND, Liu SM, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, et al. High Amylose Resistant Starch Diet Ameliorates Oxidative Stress, Inflammation, and Progression of Chronic Kidney Disease. Plos One. 2014;9(12). ARTN e114881 10.1371/journal.pone.0114881 WOS:000347515300090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–6. Epub 2015/12/04. 10.1038/nature15766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouter KE, van Raalte DH, Groen AK, Nieuwdorp M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology. 2017;152(7):1671–8. Epub 2017/02/14. 10.1053/j.gastro.2016.12.048 . [DOI] [PubMed] [Google Scholar]
- 36.Nazzal L, Roberts J, Singh P, Jhawar S, Matalon A, Gao Z, et al. Microbiome perturbation by oral vancomycin reduces plasma concentration of two gut-derived uremic solutes, indoxyl sulfate and p-cresyl sulfate, in end-stage renal disease. Nephrol Dial Transplant. 2017;32(11):1809–17. Epub 2017/04/06. 10.1093/ndt/gfx029 . [DOI] [PubMed] [Google Scholar]
- 37.Poesen R, Mutsaers HA, Windey K, van den Broek PH, Verweij V, Augustijns P, et al. The Influence of Dietary Protein Intake on Mammalian Tryptophan and Phenolic Metabolites. PLoS One. 2015;10(10):e0140820 Epub 2015/10/16. 10.1371/journal.pone.0140820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vernocchi P, Del Chierico F, Putignani L. Gut Microbiota Metabolism and Interaction with Food Components. Int J Mol Sci. 2020;21(10). Epub 2020/05/28. 10.3390/ijms21103688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eloot S, Van Biesen W, Roels S, Delrue W, Schepers E, Dhondt A, et al. Spontaneous variability of pre-dialysis concentrations of uremic toxins over time in stable hemodialysis patients. PLoS One. 2017;12(10):e0186010 Epub 2017/10/11. 10.1371/journal.pone.0186010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Log-transformed serum levels of indoxyl sulfate (A) and p-cresyl sulfate (B) are correlated with eGFR in 604 children with chronic kidney disease at study entry. Linear regression of log-transformed serum levels of indoxyl sulfate (IS) and p-cresyl sulfate (pCS) showed a significant correlation with eGFR (Pearson correlation coefficient r = -0.46 for IS and r = -0.43 for pCS). 45 patients had IS levels above the 95% upper prediction limit (UPL). While no patients had pCS levels above the 95% UPL, some patients had pCS levels below the 95% lower prediction limit.
(PDF)
Data shown as mean ± standard deviation, median (interquartile range) or n (%) as appropriate. p-value indicates group difference between consolidated cohort and those patients with IS levels exceeding the 95% UPL. IS = indoxyl sulfate; UPL = upper prediction limit; CAKUT = congenital anomalies of the kidney and urinary tract; Post-AKI = chronic kidney disease after acute kidney injury; BMI = body mass index; eGFR = estimated glomerular filtration rate; uPCr = Urinary protein creatinine ratio; SDS = standard deviation score; Non-Ca phosphate binders = non calcium containing phosphate binders; Ca phosphate binders = calcium containing phosphate binders. Countries: GER = Germany; CH = Switzerland; AT = Austria; FR = France; IT = Italy; PT = Portugal; PL = Poland; LT = Lithuania; CZ = Czech Republic; SRB = Serbia; UK = United Kingdom.
(PDF)
Diagnostic groups were compared to patients with the diagnosis of CAKUT. Residency was defined as living in Mediterranean or Non-Mediterranean countries. Physical activity was compared to being physically inactive (0 hours). SE = standard error; CAKUT = congenital anomalies of the kidney and urinary tract; Post-AKI = chronic kidney disease after acute kidney injury; BMI = body mass index; eGFR = estimated glomerular filtration rate; Ca-based P binders = calcium-based phosphate binders; uPCr = Urinary protein creatinine ratio; SDS = standard deviation score.
(PDF)
Survival analysis using the PHREG SAS procedure. If the event occurred between two visits the later of the two visits was taken as occurrence of the event instead of using linear interpolation. Model 0 represents a Cox model with sex, age, diagnosis, region of residence, body mass index SDS, blood pressure SDS, estimated glomerular filtration rate, urinary protein creatinine ratio, hemoglobin, serum phosphorus and albumin as covariates, calculated either for the total cohort (n = 569) or for the consolidated cohort (excluding patients with IS levels exceeding the 95% upper prediction limit, n = 526). Model 1a includes serum IS, 1b IS quartiles, 1c serum pCS and 1d serum pCS quartiles as a covariate to model 0. Models 1e and 1 f include serum IS and serum IS quartiles respectively as covariates to model 0 in the consolidated cohort. IS and pCS are used as logarithmic values in all models. For all variables associated with renal survival (model 0, total cohort) please refer to Table 3. IS = indoxyl sulfate; pCS = p-cresyl sulfate; Q = quartiles of serum IS and pCS levels, respectively.
(PDF)
Survival analysis for interval-censored data using the ICPHREG SAS procedure. Model 0 represents a Cox model with sex, age, diagnosis, region of residence, body mass index SDS, blood pressure SDS, estimated glomerular filtration rate, urinary protein creatinine ratio, hemoglobin, serum phosphorus and albumin as covariates, calculated either for the total cohort (n = 569) or for the consolidated cohort (excluding patients with IS levels exceeding the 95% upper prediction limit, n = 526). Model 1a includes serum IS, 1b IS quartiles, 1c serum pCS and 1d serum pCS quartiles as a covariate to model 0. Models 1e and 1 f include serum IS and serum IS quartiles respectively as covariates to model 0 in the consolidated cohort. IS and pCS are used as logarithmic values in all models. For all variables associated with renal survival (model 0, total cohort) please refer to Table 3. IS = indoxyl sulfate; pCS = p-cresyl sulfate; Q = quartiles of serum IS and pCS levels, respectively.
(PDF)
CAKUT = congenital anomalies of the kidney and urinary tract.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.