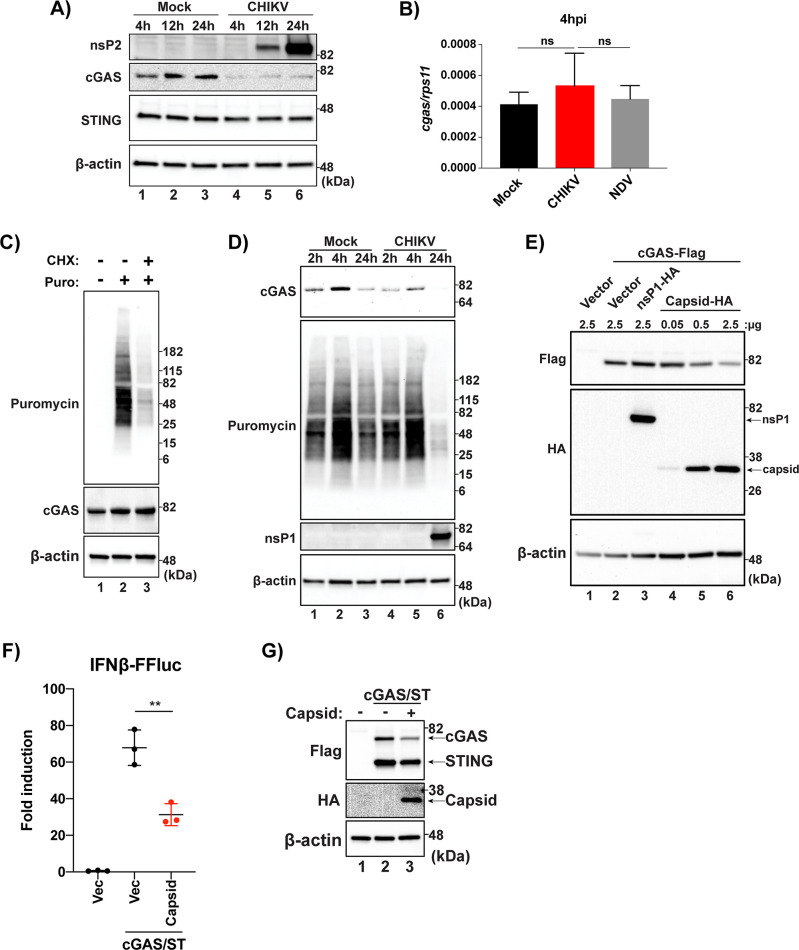
Fig 4.
(A) HFF-1s were infected with either mock or CHIKV 181/25 at an MOI of 0.1 and cell lysates were collected 4, 12, and 24 hpi. Analysis of protein from mock or CHIKV infected cells was performed via SDS-PAGE followed by immunoblotting for indicated proteins (data representative of three independent experiments). (B) RT-qPCR for cgas transcripts from HFF-1s infected with CHIKV (MOI 0.1), NDVB1 (MOI 0.1), or mock at 4hpi (data representative of three independent experiments. Data are represented by means ± SD (n = 3). Statistical analysis was done with student’s t tests. (C) HFF-1s treated with mock or CHX at 200 μg/ml for 5hrs then treated with mock or puromycin at 10 μg/ml for 15 min. After 15 min puromycin pulse, cells were washed with dPBS and re-fed complete media. Lysates collected and visualized via SDS-PAGE and immunoblotting (data representative of two independent experiments). (D) HFF-1s were infected with mock or CHIKV at an MOI of 1.0. 45 min prior to indicated timepoints, cells were pulsed with puromycin as described in (C). Lysates were collected and proteins were detected as previously stated (data representative of two independent experiments). (E) HEK-293Ts were transfected with indicated constructs at indicated plasmid amounts. Cells were allowed to rest for 24 hr post transfection before lysis and SDS-PAGE/immunoblotting analysis (data are representative of three independent experiments). (F) 293T-IFNb-FFluc cells were transfected with empty vector (Vec), cGAS and STING in conjunction with Vec, or cGAS and STING with the capsid of CHIKV 181/25. Cells were allowed to rest for 36hrs before lysis for collection of protein or quantification of luminescence. data are representative of six independent experiments. Data represented as fold induction over Vector alone. Data are represented by means ± SD (n = 3). Statistical analysis was done with student’s t tests (** = p<0.01)). (G) Protein input for IFN reporter assay in (F).