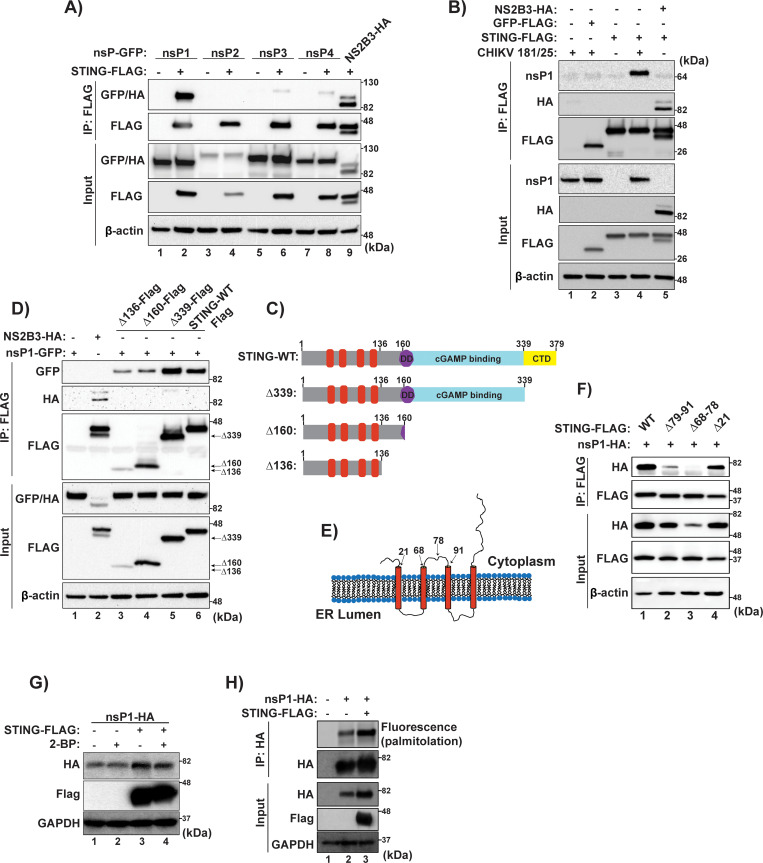
Fig 6. CHIKV nsP1 interacts with and is stabilized by STING.
(A) Flag-tagged STING was co-expressed in HEK-293T cells, individually with nsPs 1–4 of CHIKV-RT. 24 hpt, cells were lysed and an immunoprecipitation preformed against the Flag-epitope. Protein interactions were visualized via SDS-PAGE followed by immunoblotting (data representative of two independent experiments). (B) HEK-293T cells were transfected with indicated constructs and allowed to rest for 16 hrs. After resting, cells were infected with either mock or CHIKV 181/25 (MOI = 5.0). 12 hpi cells were lysed and an immunoprecipitation preformed against a Flag epitope. Protein interaction was analyzed via SDS-PAGE followed by immunoblotting (data representative of two independent experiments). (C) Diagram of STING deletion constructs used for mapping nsP1-STING interactions. (D) HEK-293Ts were transfected with CHIKV-RT nsP1 and the different STING mutants indicated in (C) and cells were lysed 24 hrs post transfection. An immunoprecipitation was performed against the Flag epitope and protein samples were then analyzed via SDS-PAGE and immunoblotting performed as described previously (data representative of two independent experiments). (E) Schematic of STING inserted in the ER membrane highlighting regions deleted which are located in cytosolic facing domains (schematic representative of poor artistic skill). (F) 293T cells were transfected with internal deletion STING constructs and nsP1. Cells were lysed 24 hpt and an anti-flag IP was performed followed by SDS-PAGE and immunoblotting (data representative of three independent experiments). (G) HEK-293T cells were transfected cells with indicated constructs overnight followed by treatment with 100 uM 2-BP for 24 h. Cells were lysed and analyzed via Western blotting. (H) HEK-293T cells were transfected with indicated constructs overnight and treated for 1 h with 20 uM alk-16 palmitoylation chemical reporter reagent prior to cell lysis. Immunoprecipitation was performed against the HA epitope followed by click chemistry reaction with azido-rhodamine for visualization of protein palmitoylation via fluorescence gel scanning. (G & H) data representative of two independent experiments.