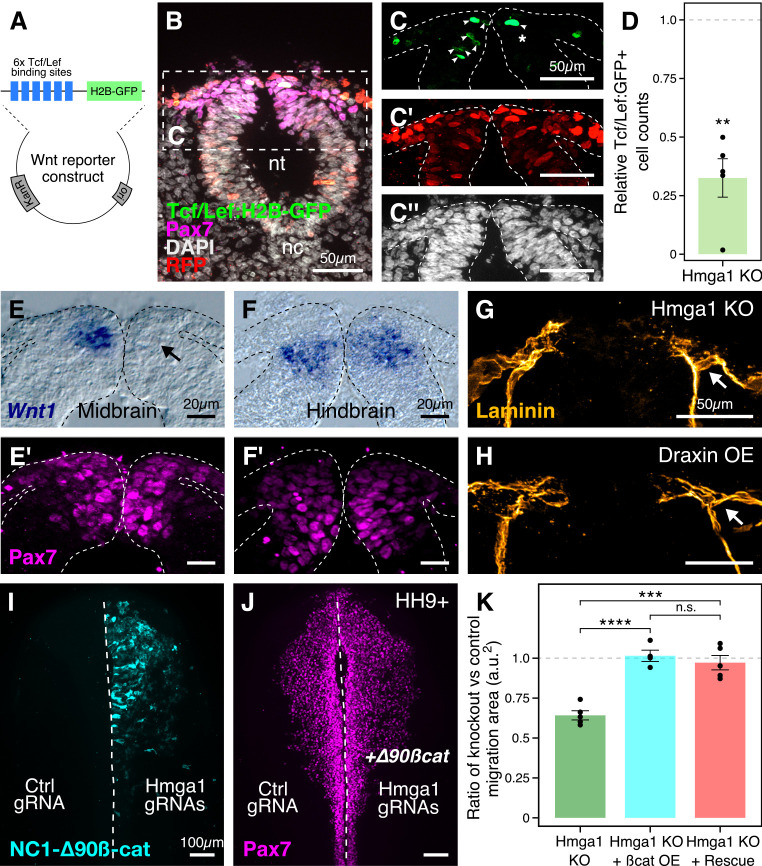
Figure 6. Hmga1 activates Wnt signaling pathway in delaminating neural crest cells.
(A) Plasmid construct used as a readout for Wnt activity (after Ferrer-Vaquer et al., 2010). Six TCF/Lef-binding sites together with a minimal promoter regulate the expression of nuclear GFP in transfected cells in response to Wnt signaling. (B) Transverse section through the midbrain of a representative HH9+ embryo immunostained for Pax7, GFP, RFP, and DAPI. (C–C’’) Individual channels of image in B focused on the dorsal neural tube. In the absence of Hmga1, Wnt reporter activity was downregulated, resulting in fewer cells that expressed nuclear GFP (arrowheads) on the right side (C), even though cells were uniformly transfected on both experimental and control sides (C’), and the thickness of the neural tube remained unaffected (C’’). (D) The reduction in Wnt reporter output was quantified as a ratio of number of cells that expressed nuclear GFP, and the number of cells that were successfully transfected and therefore expressed nuclear RFP. The observed difference in GFP+/RFP+ ratio between the knockout and control sides was statistically significant (**p<0.01, student’s t-test). (E–F) In situ hybridization against Wnt1 in an Hmga1-knockout background. Transverse section through the midbrain (E) and hindbrain (F) shows reduced and unchanged levels of Wnt1 ligand in the dorsal neural tube on the experimental (arrow) versus control neural tubes, respectively. (E’–F’) The number of Pax7-positive cells in the midbrain appeared unchanged (E’), while a reduction was observed in the hindbrain (F’). (G–H) Transverse section through a representative embryo where Hmga1 was knocked out using CRISPR plasmids (G), and an embryo where Draxin was ectopically expressed (H) on the right side, immunostained for Laminin. Similar to Draxin overexpression, Hmga1 loss resulted in a failure of basement membrane remodeling due to reduced canonical Wnt signaling in neural crest cells, causing the laminin channel to remain blocked on the experimental side (arrows). (I–J) Expression of stabilized ß-catenin (NC1-∆90ß-cat) in delaminating cranial neural crest of Hmga1-knockout embryos was sufficient to rescue the migration defect. (K) Quantification of area covered by cranial neural crest cells on the experimental versus control sides. In the absence of Hmga1, cranial neural crest cells fail to migrate properly, a defect that can be separately rescued in Hmga1-knockout background by overexpression (OE) of stabilized ß-catenin in delaminating cranial neural crest, or exogenous expression of Hmga1 coding sequence ectopically (Rescue). nt, neural tube; nc, notochord; KO, knockout; OE, overexpression. See also Figure 6—figure supplement 1, Figure 6—source data 1, Figure 6—source data 2.