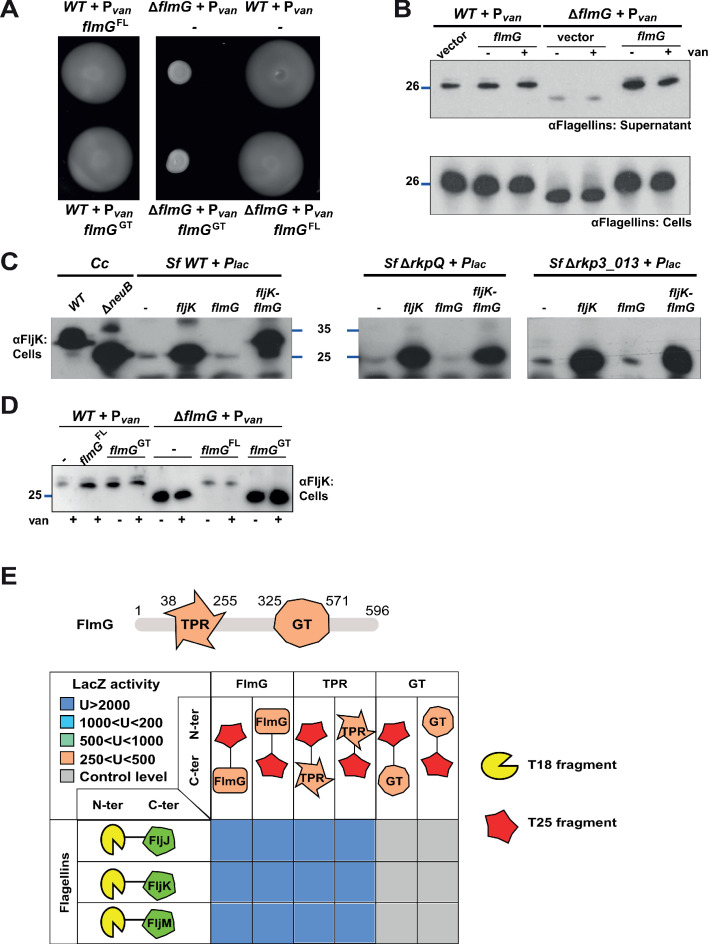
Figure 4. FlmG is the putative glycosyltransferase required to post-translationally modify flagellins.
(A) Motility assay of WT and ΔflmG cells complemented with Pvan-flmG full-length (FL) or glycosyltransferase CTD (GT) on plasmid. ΔflmG cells are non-motile, as indicated by the compact swarming. The expression of FlmG full-length from Pvan restores motility, in contrast to the GT domain alone. (B) Immunoblot showing the levels of flagellins in supernatants and whole cell lysates from WT and ΔflmG cells complemented with Pvan-flmG on a plasmid. Flagellins produced by ΔflmG cells show the same migration profile as those produced by ΔneuB cells, with faster migration and reduced abundance in the supernatant. The blue lines on the left indicate the molecular size standard, with the corresponding value in kDa. Note that antibodies used in this blot were raised against flagellins purified from C. crescentus (αFlagellins; Hahnenberger and Shapiro, 1987). (C) Immunoblot on C. crescentus FljK expressed in S. fredii NGR234 strains. The left panel shows that when expressed alone in WT S. fredii FljK migrates faster, like in the C. crescentus ΔneuB mutant. By contrast, co-expression of FlmG and FljK in S. fredii NGR234 results in a shift in the migration profile of FljK, similar to that observed in C. crescentus WT cells. When FlmG and FljK are co-expressed in S. fredii strains unable to synthesize pseudaminic acid (ΔrkpQ, middle panel; Δrkp3_013, right panel) FljK shows only the fast migrating band, independently from the presence of FlmG. The blue lines indicate the molecular size standards, with the corresponding value in kDa. (D) Immunoblot with anti-FljK antibodies on whole cell lysates from WT and ΔflmG cells expressing FlmG full-length (FL) or the glycosyltransferase CTD (GT) from Pvan on a plasmid. The expression of the GT domain alone does not restore the migration profile of flagellins in ΔflmG cells, in agreement with the motility defect shown in panel A. (E) BACTH assay showing the interaction of FlmG with flagellins. The cartoon represents FlmG, with the N-terminal TPR domain and the C-terminal glycosyltransferase domain. The scheme below shows the β-galactosidase activity, expressed in Miller units (U) of E. coli BTH101 cells containing the pair-wise combinations of the different constructs used for the BACTH assay. T18 and T25 correspond to the two fragments of the adenylate cyclase and are represented as yellow shape and red star, respectively. Hybrids with the proteins of interest (FlmG, FljJ, FljK, and FljM) were created as N-ter or C-ter fusions, as mentioned. In the case of FlmG, fusions were created with the full-length protein, the TPR domain or the glycosyltransferase (GT) domain only. Gray squares correspond to β-galactosidase activity similar to the control (empty plasmid, U < 250), orange squares indicate an activity between 250 and 500 U, light green squares between 500 and 1000 U, light blue between 1000 and 2000 U, and dark blue above 2000 U. The values of β-galactosidase correspond to the mean and standard deviation of three independent experiments and are listed in Supplementary file 1 Table S1.