Abstract
Background:
Ambient air pollution exposure has been associated with dementia. Additionally, epidemiologic evidence supports associations between air pollution and diabetes as well as diabetes and dementia. Thus, an indirect pathway between air pollution and dementia may exist through metabolic dysfunction.
Objective:
To investigate whether local traffic-related air pollution (TRAP) influences incident dementia and cognitive impairment, non-dementia (CIND) in a cohort of older Mexican Americans. We also assess how much of this estimated effect might be mediated through type 2 diabetes (T2DM).
Methods:
In a 10-year, prospective study of Latinos (n = 1,564), we generated TRAP-NOx as a surrogate for pollution from local traffic sources at participants’ residences during the year prior to enrollment. We used Cox proportional hazards modeling and mediation analysis to estimate the effects of TRAP-NOx on dementia and/or CIND and indirect pathways operating through T2DM.
Results:
Higher TRAP-NOx was associated with incident dementia (HR = 1.55 for the highest versus lower tertiles, 95% CI = 1.04, 2.55). Higher TRAP-NOx was also associated with T2DM (OR = 1.62, 95% CI = 1.27, 2.05); furthermore, T2DM was associated with dementia (HR = 1.94, 95% CI = 1.42, 2.66). Mediation analysis indicated that 20% of the estimated effect of TRAP-NOx on dementia/CIND was mediated through T2DM.
Conclusion:
Our results suggest that exposure to local traffic-related air pollution is associated with incident dementia. We also estimated that 20% of this effect is mediated through T2DM. Thus, ambient air pollution might affect brain health via direct damage as well as through indirect pathways related to diabetes and metabolic dysfunction.
Keywords: Air pollution, CALINE4, cognitive decline, dementia, diabetes, Latino, mediation, nitrogen oxide, traffic-related air pollution
INTRODUCTION
Exposure to ambient air pollution is ubiquitous and has been associated with various adverse age-related health outcomes in adults, including cardiovascular disease and type 2 diabetes (T2DM) [1, 2]. While epidemiologic evidence is still accumulating, higher levels of long-term exposure has also been repeatedly associated with cognitive dysfunction in the elderly, including incident cognitive impairment and dementia [3–8]. This includes two large cohorts, the Betula study in Northern Sweden and the Women’s Health Initiative Memory Study (WHIMS), which both reported that residential air pollution increased incident dementia risk [4, 5]. Among adults over 65, the prevalence of mild cognitive impairment and of dementia is thought to be 30–40% and 10%, respectively; and both are associated with considerable personal and societal burdens [9]. These previous, high-quality, prospective longitudinal studies have estimated the population attributable fraction of ambient air pollution exposure to dementia (Alzheimer’s or vascular type) at between 16 and 21% [4, 5].
However, while abundant evidence supports differential racial/ethnic vulnerability to a wide array of diseases including cognitive outcomes [10–12], and racial/ethnic and neighborhood socioeconomic features are reported to co-vary with air pollution [13, 14], the majority of prospective, longitudinal cognitive research has been conducted primarily in non-Hispanic, white populations. The Sacramento Area Latino Study on Aging (SALSA), a large, prospective cohort study of community-dwelling Latino Americans, therefore, is a unique resource for examining the influence of air pollution on incident dementia among elderly Hispanics.
Furthermore, over the past two decades, convincing evidence has accumulated that T2DM patients are at increased risk of developing dementia later in life, relative to non-patients [15, 16]. With robust epidemiologic evidence supporting an association between ambient air pollution and diabetes [2] and a high prevalence of diabetes among Mexican Americans, it is important to not only consider the influence air pollution has on dementia directly, but also how air pollution may influence dementia risk indirectly through first affecting diabetes.
It is commonly thought that Alzheimer’s disease (AD) and vascular neuropathology synergize in contributing to cognitive impairment and dementia late in life [17–19]. Shared pathways between air pollution and T2DM have been linked to this process, with vascular and metabolic disturbances related to diabetes possibly exacerbating neuronal dysfunction and contributing to dementia [20]. Consequently, an indirect pathway between air pollution and dementia may exist through T2DM, and increases in peripheral and neuronal insulin resistance [21, 22], inflammation, mitochondrial dysfunction, and chronic oxidative stress have all been directly implicated [23–25].
In the present study, we investigated whether local traffic-related air pollution (TRAP), as measured by a surrogate criteria pollutant marker (TRAP-NOx) derived from a line source dispersion model, influences two incident cognitive outcomes, dementia and cognitive impairment (non-dementia), in a cohort of older Mexican Americans. Secondarily, we assessed how much of this estimated association might be mediated through T2DM. We apply mediation methods to separate the estimated direct effects of traffic-related air pollution from indirect effects operating through T2DM on cognitive outcomes.
METHODS
Study population
Participants were enrolled in the Sacramento Area Latino Study on Aging (SALSA), a prospective, community-based cohort study that enrolled Latino participants primarily of Mexican origins (95%). All Latino residents from California’s Sacramento Valley area aged more than 60 years were eligible to enroll (for a detailed description of sampling procedures see [26]). The overall response rate was 85% for those contacted, and 1,789 participants aged 60 to 101 years were enrolled and examined in 1998 to 1999 [26]. Cohort members were followed every 12 to 15 months via home visits. Clinical and cognitive assessments were conducted up to seven times during these home visits, ending in 2008. In a semiannual 10 min telephone call between home visits, interviewers obtained updates on medications, health events, and some additional sociodemographic factors. Participants who had cognitive impairment non-dementia (CIND) or dementia at baseline (n = 115, 6.4%), those who did not participate in any follow-up interviews (n = 57, 3.2%), or did not provide all necessary information (n = 53, 3.0%) were excluded, leaving 1,564 participants for analyses. See Supplementary Figure 1 for details on attrition and death over follow-up. The median participation was 5 visits per person (mean = 4.7, SD = 2) for the whole cohort (n = 1,789), and 6 visits (mean = 4.9, SD = 2) for the analysis population (n = 1,564)
Air pollution exposure
In this study, we focused on local-traffic generated pollution from traffic emissions at the participants’ residential address averaged over the year of enrollment.
We employed a modified California Line Source (CALINE4) dispersion model to estimate subjects’ residential TRAP-NOx exposure originating from traffic emissions within 1.5 km of participants’ residential addresses over the year of enrollment [27–29]. Previous studies showed varying but relatively narrow impact zones of primary traffic emissions, ranging from approximately 300 m during daytime [30] up to 2,600 m during pre-sunrise hours [31]; 1.5 km is the average of the two impact zone sizes.
CALINE4 is a Gaussian dispersion model that employs a mixing zone concept to characterize pollutant dispersion over the roadway. Major inputs to CALINE4 include meteorology (atmospheric stability, mixing height, wind, and temperature), roadway geometry and traffic activities, and vehicle emission factors. A comprehensive traffic database with annual average daily traffic counts and gasoline and diesel vehicle fractions was constructed for the entire Sacramento study region. Meteorological data were obtained from the California Air Resources Board (CARB) and the National Weather Service [32]. The motor vehicle emission factor model, EMFAC2011, developed by CARB was used to generate TRAP-NOx emission rates [33]. Fleet-average emission factors were calculated using county-specific vehicle registration and temperature data applying emission factors to segment-level gasoline and diesel vehicle counts on roadway networks. The final product is a yearly average TRAP-NOx concentration estimate for the year of enrollment from vehicular traffic within 1.5 km of each participant’s residence. A more in depth discussion of the model has been published [28, 29]. Our estimated pollutant (TRAP-NOx) exposure measure should be regarded as an indicator of primary emissions from local vehicular traffic within the vicinity of each participant’s residence during the year of enrollment.
We treated the average TRAP-NOx estimated exposure during the year of enrollment both as a continuous predictor, with effect estimates representing an increase per one interquartile range (IQR: 2.31 ppb), and also dichotomized at above and below the highest tertile of TRAP-NOx (>2.68 ppb versus ≤2.68 ppb).
In terms of model verification, CALINE4 was originally developed and verified by the California Department of Transportation (Caltrans) [27, 34]. The original study verified modeled estimates using data from five field studies, with measurements at a series of study sites across California and the US done in conjunction with the EPA (hundreds of measurements per site) [27, 34]. At least 75% of all paired data fell within the factor-of-two envelope window. The factor-of-two envelope, within plus or minus a factor of two of the measured concentrations, is a common minimum criterion for judging adequate model performance. For NO2, the correlation (R) between the predicted and measured concentration across the field sites ranged between 0.79 (freeways) – 0.83 (intersections). Overall, 12% of the NO2 estimates were outside of the factor-of-two envelope. Wind directional shifts were determined as the most important factor for misclassification. Another study that verified CALINE4 estimated PM2.5 in the Sacramento region, found that with the inclusion of background concentrations in the model, all paired points (measures and estimates) were inside the factor-of-two envelope. Typically, if 80% of the points fall inside the factor-of-two envelope, the model results are considered performing well in predicting true values. The correlation and R2 between measured and predicted values was also high (with background pollutant levels in the model: R2 = 0.95, without background R2 = 0.90) [35].
Furthermore, our study team (lead by J.W.) has also conducted internal comparisons of modeled versus measured data. Using comparisons based on measurements from the USC Children’s Health Study field campaign measurements, the study showed a high correlation (R = 0.87) of CALINE4-modeled monthly NOx concentrations with measurements at nine monitoring sites in the Long Beach study area in December 2007 and April 2008 (Wu J, Lurmann F, Avol E, unpublished data).
Dementia and cognitive impairment, non-dementia
All participants were screened for cognitive outcomes during each home visit. Detailed procedures to screen and classify participants with dementia and CIND are described elsewhere [26]. Briefly, each participant was assessed with two cognitive screening tests at each in-person visit (up to seven home visits), the Modified Mini–Mental State Examination (3MSE) and a delayed word recall trial from the Spanish English Verbal Learning Test (SEVLT). Participants were referred for a neuropsychological test battery [36] and a standard neuropsychological examination (Informant Questionnaire on Cognitive Decline in the Elderly) if (1) their scores on the 3MSE or SEVLT fell below the 20th percentile, or (2) had decreased from baseline by ≥8 points (3MSE) or ≥3 points (SEVLT) [26]. A team of neurologists and a neuropsychologist reviewed all referred cases and classified each as demented, CIND, or cognitively normal. Standard diagnostic criteria were applied for a diagnosis of dementia [37].
Type 2 diabetes
T2DM status was based on reports of a physician diagnosis, inventory of antidiabetic medication use, or measured fasting glucose level ≥126 mg/dL (7.0 mmol/l) in a blood sample. We assessed prevalent T2DM as the mediator between air pollution and the cognitive outcomes, for two primary reasons, 1) diabetes was highly prevalent in the SALSA population at baseline (n = 500, 32%; Table 1); 2) previous work in SALSA and other studies has demonstrated that those with prevalent diabetes at baseline displayed faster cognitive decline, while those who developed incident diabetes (n = 181, 12%) over follow-up showed similar cognitive decline to those without diabetes (n = 883, 56%) [38]. Thus, while the lack of an association between incident diabetes in SALSA is most likely due to an insufficient latency period between diabetes onset and cognitive events, incident diabetes cannot be treated as a mediator as it is not related to the outcome. We also assume that air pollution measured during the year of enrollment is representative of the long-term relative levels of air pollution at residences, providing a basis for temporality. This is reasonable as the majority of the SALSA population reported living at their baseline address for many years prior to enrollment (mean = 21.8 years; median 23.8 years; range 0.3 to 74 years; IQR = 10.5 to 30 years; 90% >4 years). Furthermore, we present this secondary mediation aim as first epidemiologic evidence linking air pollution, diabetes, and subsequent dementia, and hope other cohorts with longer follow-up and incident diabetes further assess these pathways to provide evidence for temporality.
Table 1.
Population characteristics of SALSA participants at baseline by dementia/ CIND status established during follow-up.
| SALSA analysis population | No Dementia/CIND | CIND | Dementia | Dementia/CIND | |
|---|---|---|---|---|---|
| Variable, Mean ± SD/n (%) | N=1564 | N=1410 | N=67 | N=100 | N=154 |
| Age (y) | 70.2 ± 6.8 | 69.7 ± 6.8 | 73.8 ± 7.8* | 76.7 ± 7.6* | 75.4 ± 7.7* |
| Male | 656 (41.9) | 598 (42.4) | 25 (37.3) | 36 (36.0) | 58 (37.7) |
| Primarily Language: Spanish | 886 (56.7) | 791 (56.1) | 43 (64.2) | 61 (61.0) | 95 (61.7) |
| Education (y) | 7.3 ± 5.3 | 7.5 ± 5.3 | 6.0 ± 5.9* | 5.5 ± 4.4* | 5.7 ± 5.1* |
| Cigarette Smoking, Current/Former | 745 (47.6) | 666 (47.2) | 36 (53.7) | 51 (51.0) | 79 (51.3) |
| Occupation | |||||
| Non-manual | 341 (21.8) | 328 (23.3) | 13 (8.4)* | 8 (8.0)* | 13 (8.4)* |
| Manual | 944 (60.4) | 841 (60.0) | 103 (66.9)* | 68 (68.0)* | 103 (66.9)* |
| Other (housewives and unemployed) | 279 (17.8) | 241 (17.1) | 38 (24.7)* | 24 (24.0)* | 38 (24.7)* |
| Urban Residence | 1359 (86.9) | 1228 (87.1) | 58 (86.6) | 86 (86.0) | 131 (85.0) |
| CALINE4 TRAP (NOx) ppb (mean) | 2.59 ± 2.2 | 2.58 ± 2.2 | 2.81 ± 2.5 | 2.65 ± 2.2 | 2.68 ± 2.2 |
| ≤ 2.68 (T3) ppb | 1034 (66.1) | 936 (66.4) | 44 (66.1) | 63 (63.0) | 98 (63.6) |
| >2.68 ppb | 530 (33.9) | 474 (33.6) | 23 (34.3) | 37 (37.0) | 56 (36.4) |
| NSES (1 low to 5 high) | 2.09 ± 1.0 | 2.10 ± 1.0 | 1.96 ± 1.0 | 2.10 ± 1.9 | 2.00 ± 1.0 |
| CA Residential County | |||||
| Sacramento | 1221 (78.1) | 1108 (78.6) | 50 (74.6) | 76 (76.0) | 113 (73.4) |
| Yolo | 199 (12.7) | 172 (12.2) | 10 (14.9) | 17 (17.0) | 27 (17.5) |
| Other† | 144 (9.2) | 130 (9.2) | 7 (10.4) | 7 (7.0) | 14 (9.1) |
| Baseline type 2 diabetes | 500 (32.0) | 432 (30.6) | 30 (44.8)* | 45 (45.0)* | 68 (44.2)* |
| Baseline 3MSE | 85.9 ± 11.5 | 86.7 ± 78.6* | 82.1 ± 11.1* | 76.5 ± 17.8* | 78.6 ± 16.0* |
P-value<0.05 based on t-test or chi-square, compared to no dementia/CIND group
Solano, Calaveras, Placer, Stanislaus, Sutter, Yuba
CALINE4= California Line Source dispersion model 4; CVD=cardiovascular disease; CIND=cognitive impairment, non-dementia; ppb=parts per billion; T3=tertile 3; T2DM=type 2 diabetes mellitus; TRAP=traffic-related air pollution;
Neighborhood socioeconomic score
At baseline, participants were residing in 251 different census tracts, each containing on average 6 participants (range 1–71 participants). We generated a neighborhood socioeconomic status score (NSES) for each census block, and, thus, for each residence, based on the method developed by Yost et al. [39]. To assess NSES information, census variables were aggregated at the census block group level, as blocks contain approximately one-fourth the individuals in a census tract (∼1000 individuals), and are generally more homogenous related to SES factors, and represent smaller geographic areas than census tracts [39].
The NSES composite score was created from the 2000 census and SALSA participants’ residential address information. More detail has been published [39]. Briefly, seven census derived variables were combined into a single NSES index using principal component analysis. These included: 1) an education index that weights proportion of people in the census block with a given education level by years to attain that education; 2) proportion with a blue-collar job; 3) proportion older than 16 in the workforce without a job; 4) median household income; 5) proportion below 200% of the poverty level; 6) median rent; and 7) median house value. Higher values of variables 1, 4, 6, and 7 represent higher SES, and these variables had positive loadings into the component. While variables 2, 3, and 5 are inversely related to SES and had negative loadings into the component. Thus, a larger value on the NSES component represents a higher NSES, and the score ranges from 1 (low NSES) to 5 (high NSES). Each census block was scored based on this method, and individuals assigned an NSES score based on the score of the census block they lived in at baseline.
Statistical methods
Our primary aim was to assess the relationship between TRAP-NOx and incident dementia and incident CIND. We therefore, present three sets of estimates, for TRAP-NOx and time to dementia, time to CIND, and time to dementia or CIND, whichever was diagnosed first (dementia/CIND). We used Cox proportional hazards models for time to event analysis, clustered by census tract (i.e., the standard errors of the effect estimates are corrected for the clustering of participants within census tracts). To control for confounding of the exposure-outcome association, we added to the models baseline age, sex, years of education, primary language spoken (English/Spanish), occupation for most of the participant’s life (manual, non-manual, or other (housewife or unemployed)), smoking status (current/former, never), NSES, an indicator for participants living in one of the six smaller counties (yes/no), an indicator of urbanicity (rural/urban) within each county, and baseline cognitive function (3MSE score). We also present sensitivity analyses excluding 3MSE score from the models.
In order to assess deviations from linearity when using the TRAP-NOx exposure per IQR, we additionally fit a series of Cox proportional hazards models allowing for splines, and we selected a cubic spline with 3 knots as it fit the data best. We then compared the original Cox proportional hazards models without splines, by cognitive outcome, to the model with the cubic spline with a likelihood ratio test.
Urbanicity is strongly related to TRAP-NOx, that is from among the 205 participants residing in rural locations during the year of study enrollment, only 10 had a TRAP-NOx exposure in the highest tertile (Supplementary Table 1). Thus, we present results based on the entire SALSA cohort (n = 1,564) and also only for those living in urban environments only (n = 1,359). Differences in the distribution of baseline demographics and health characteristics by dementia/CIND status over follow-up were compared using the Student’s t-test (continuous data) and Pearson chi-square test (categorical data).
We also conducted additional sensitivity analysis, to assess whether loss to follow-up over the study period was informative. We first assessed whether any of our covariates or exposures were predictive of censoring due to loss to follow-up (for any reason) using logistic regression. To account for the possibility of selection bias from right censoring, we then used an inverse probability weight for censoring (IPCW) to create a pseudo-population mimicking the total population before censoring in distribution of measured covariates and potential predictors of loss to follow-up [40]. We then ran the Cox models in the uncensored population only (those who developed incident cognitive outcomes or completed 4 follow-up exams), but included the IPCW weight.
Our secondary aim was to assess the indirect association of TRAP-NOx on the incident cognitive outcomes operating through prevalent T2DM. In previous work, prevalent T2DM has been shown to be associated with cognitive outcomes in SALSA [38]. Thus, as the first step to assess if mediation analysis is suitable, we assessed whether TRAP-NOx was associated with prevalent T2DM using logistic regression with census-tract clustered standard errors. The clustering in the logistic regression models was done using generalized estimated equations (GEE), which is a regression approach for generalized linear models when the responses are correlated/clustered. We used the geeglm function (geepack), including the census tract as id.
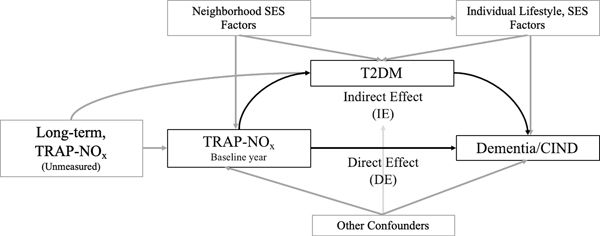
Once we confirmed that TRAP-NOx is associated with T2DM, we employed mediation analysis to estimate the natural direct and indirect effects. Figure 1 details the modeled presumed pathways of effect between TRAP-NOx and dementia/CIND. To assess the natural direct and indirect effects of TRAP-NOx operating through T2DM, we performed mediation analysis using an approach proposed by Lange et al. [41] based on marginal structural models that directly parameterize the natural direct and indirect effects of interest.
Fig. 1.
Proposed mediation analysis pathways: relationship between traffic-related air pollution, type 2 diabetes, and dementia/cognitive impairment non-dementia (CIND).
The approach utilizes the counterfactual framework [42] and allows decomposition of the total effect of the exposure (TRAP-NOx) on the outcome (dementia) into a natural direct effect (TRAP-NOx → dementia) and natural indirect effect through a mediator (TRAP-NOx → T2DM → dementia). We calculated natural effects to allow for the potential presence of exposure-mediator interactions. This method involves four main steps: 1) Estimation of the effects of TRAP-NOx exposure on the mediator (T2DM) with a logistic regression model. 2) Constructing a new data set repeating each observation from the original data set twice and including an additional variable TRAP-NOx*, which is equal to the original “observed” exposure for the first replication of the original dataset but equal to the counterfactual “unobserved” exposure for the second replication. For the dichotomized TRAP-NOx exposure this is the opposite of the observed exposure level. 3) Then we computed weights for each observation by applying the fitted model from step 1, to distinguish between the direct and indirect paths (W = P(M | TRAP-NOx*, C)/P(M | TRAP-NOx, C), where M = mediator and C = confounders). And 4) with the new data set in place, we fitted a weighted Cox proportional hazards model with W (step 3) that includes TRAP-NOx and TRAP-NOx *. We allowed for an interaction between exposure and the mediator by including a product term in the model, but since we did not estimate any interactions (OR = 0.96–1.03) or meaningful change in effect (TE, IE, DE) we did not include this term in the final model.
Assuming proportional hazards, the hazard ratios for TRAP-NOx and TRAP-NOx* serve as estimates for the natural direct effect (DE) and indirect effect (IE) of TRAP-NOx and dementia/CIND. The product of the two hazard ratios yields the hazard ratio for the total effect (TE) and the proportion mediated is IE/TE. Confidence intervals that account for clustering within census tracts were obtained using 5,000 bootstrap simulations (2.5 and 97.5 percentiles). The mediation method assumes no unmeasured confounding, not only for the exposure-outcome, but also exposure-mediator and mediator-outcome relationships. Thus, we also included an inflammation marker (IL-6; n = 160 participants missing this value and excluded from the mediation analysis) in the exposure-outcome model, since inflammation can be considered a possible confounder of the mediator-outcome association that is influenced by the exposure.
We include R code for analysis in the supplement for ease of replication, though we encourage readers to see Lange et al. [41] and Rochon et al. [43] for a more detailed discussion on the mediation approach and coding using R. Analyses were conducted using R version 3.4.4 [44], packages lme4, geepack, and survival. Analysis code can be found in the Supplementary Material.
RESULTS
Baseline demographics and health characteristics of the SALSA population are presented in Table 1. Participants who developed dementia or CIND during follow-up were on average older at baseline (75.4 (SD = 7.7) versus 69.7 (SD = 6.8)), had a higher proportion of cases who were previously employed as manual laborers (67% versus 60%), had less years of education (5.7 (SD = 5.1) versus 7.5 (SD = 5.3)), and a higher proportion had T2DM (44% versus 31%) at baseline (Table 1). The majority of the study population (87%) resided in urban locations, where estimates of local TRAP-NOx exposure were on average higher than in rural locations (2.8 ppb (SD = 2.2) versus 1.2 ppb (SD = 0.7); Supplementary Table 1). The level of estimated local TRAP-NOx also generally decreased with increasing NSES (p = 0.03; Supplementary Figure 2).
During the 10-year follow-up period, 154 (9.8%) participants developed either incident dementia or CIND. A total of 67 (4.2%) participants were diagnosed with incident CIND; of these, 13 were subsequently diagnosed with dementia during follow-up, while 54 were censored. A total of 100 (6.4%) participants were diagnosed with incident dementia, 87 were diagnosed with only dementia, while 13 had been diagnosed with CIND first at a previous visit. Among those with incident dementia/CIND, the median age at event was 80.4 years and time from baseline to event, either CIND or dementia whichever was diagnosed first, was 5.1 years.
The association between TRAP-NOx and the cognitive outcomes can be found in Table 2. Higher levels of residential TRAP-NOx, averaged over the year of enrollment, were associated with a faster time to dementia (HR = 1.55 for the highest versus lower tertiles, 95% CI = 1.04, 2.33; HR = 1.20 per IQR (2.31 ppb), 95% CI = 0.98, 1.47). The magnitude of association was slightly stronger when we assessed associations among those living in urban environments only (HR = 1.65 for the highest versus lower tertiles, 95% CI = 1.10, 2.48; HR = 1.25 per IQR (2.31 ppb), 95% CI = 1.03, 1.52). For time to CIND, excluding from the control group those who were diagnosed with dementia but not CIND (n = 87), the magnitude of the association for dichotomized TRAP-NOx was attenuated (HR = 1.21 for the highest versus lower tertiles, 95% CI = 0.76, 1.92), while TRAP-NOx, per IQR showed a similar size association as dementia (HR = 1.21 per IQR, 95% CI = 1.00, 1.46). Time to dementia or CIND, whichever was first diagnosed, was also increased (HR = 1.45 for the highest versus lower tertiles, 95% CI = 1.07, 1.96; HR = 1.21 per IQR, 95% CI = 1.05, 1.40). We also assessed these associations excluding baseline cognitive scores (3MSE) from the models, as TRAP-NOx likely represents longer-term exposure levels, and thus may influence baseline cognitive function, acting as a mediator versus acting as a confounder. These results can be found in Supplementary Table 2; and the findings were generally quite similar.
Table 2.
Relationship between TRAP-NOx and late-life incident cognitive outcomes.
| Outcome | TRAP-NOx Exposure Category | All SALSA (n=1564) |
Urban Only (n=1359) |
||||
|---|---|---|---|---|---|---|---|
| Cognitive Outcome |
HR (95% CI) |
Cognitive Outcome |
HR (95% CI) |
||||
| No (%) | Yes (%) | No (%) | Yes (%) | ||||
| Time to incident dementia | NOx T3 (yes/no) | ||||||
| ≤ 2.68 ppb | 971 (66.3) | 63 (63.0) | 1.00 (ref) | 790 (62.1) | 49 (57.0) | 1.00 (ref) | |
| 100/86 (urban only) events | >2.68 ppb | 493 (33.7) | 37 (37.0) | 1.55 (1.04, 2.33) | 483 (37.9) | 37 (43.0) | 1.65 (1.10, 2.48) |
| per IQR | 1.20 (0.98, 1.47) | 1.25 (1.03, 1.52) | |||||
| Time to incident CIND† | NOx T3 (yes/no) | ||||||
| ≤ 2.68 ppb | 936 (66.4) | 44 (65.7) | 1.00 (ref) | 764 (62.2) | 35 (60.3) | 1.00 (ref) | |
| 67/58 (urban only) events | >2.68 ppb | 474 (33.6) | 23 (34.3) | 1.21 (0.76, 1.92) | 464 (37.7) | 23 (39.7) | 1.26 (0.79, 2.03) |
| per IQR | 1.21 (1.00, 1.46) | 1.24 (1.02, 1.50) | |||||
| Time to incident dementia/CIND | NOx T3 (yes/no) | ||||||
| ≤ 2.68 ppb | 936 (66.4) | 98 (64.6) | 1.00 (ref) | 764 (62.2) | 75 (57.3) | 1.00 (ref) | |
| 154/131 (urban only) events | >2.68 ppb | 474 (33.6) | 56 (36.4) | 1.45 (1.07, 1.96) | 464 (37.8) | 56 (42.8) | 1.55 (1.15, 2.09) |
| per IQR | 1.21 (1.05, 1.40) | 1.25 (1.09, 1.44) | |||||
Abbreviations: CIND=Cognitive Impairment, non-dementia; T3=tertile 3 (TRAP NOx dichotomized at >T3); IQR=interquartile range (2.31 ppb)
Based on Cox proportional hazards regression with census-tract clustered standard errors.
Models control for baseline 3MSE score, baseline age, sex, years of education, primary language spoken, occupation for most of life (manual, non-manual, or other), NSES, cigarette smoking, a county indicator, and a rural/urban residential neighborhood indicator (excluded for urban only analysis).
Excludes those who were diagnosed with dementia (but not CIND) from the control group
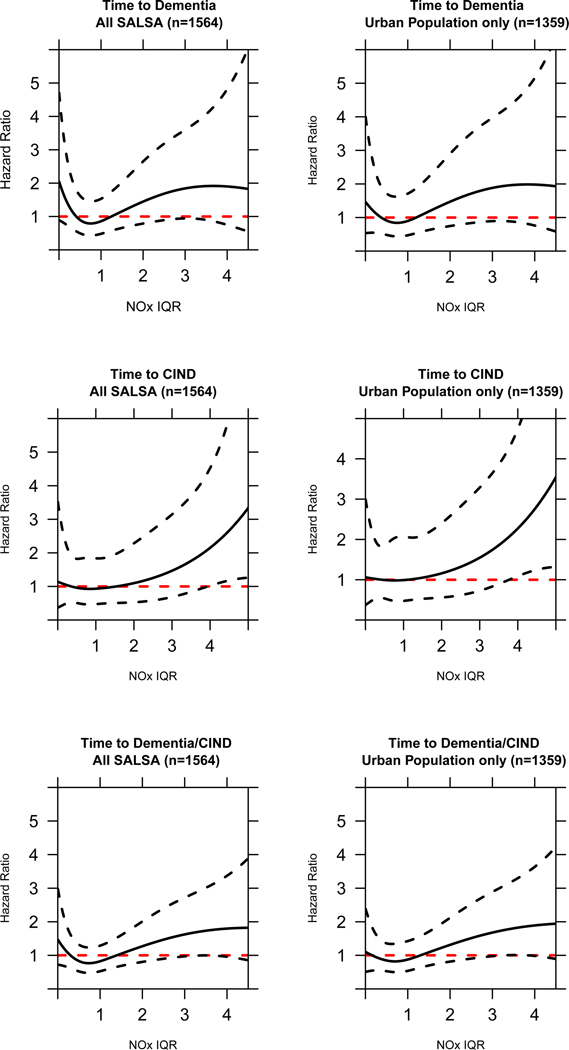
We also assessed non-linearity of the TRAP-NOx (per IQR) association with splines (cubic spline, 3 knots). When comparing the models without to those with the splines, none of the models showed statistically significant differences in fit (likelihood ratio test: Time to dementia, All SALSA p = 0.12, Urban only p = 0.41; Time to CIND, All SALSA p = 0.63, Urban only p = 0.73; Time to dementia/CIND, All SALSA p = 0.16, Urban only p = 0.55). The spline models can be viewed in Fig. 2, which demonstrates that TRAP-NOx (per IQR) and time to CIND was quite linear. For time to dementia, there appears to be some leveling off of risk at higher exposure profiles (TRAP-NOx IQR > 2.5). Additionally, for time to dementia, in the analysis of the total population, there is some increase of risk displayed for those with the lowest risk profiles as well. This is driven by rural participants, as a majority with very low TRAP-NOx profiles (<0.5) lived in rural environments: 75% of those with <0.5 TRAP-NOx are from rural environments, while those with higher exposure profiles lived in more urban environments.
Fig. 2.
Relationship between TRAP-NOx (per IQR) and late-life incident cognitive outcomes: allowing for non-linear associations with a cubic spline (3 knots). The results are displayed for each outcome based on the whole SALSA cohort and limiting to those in urban environments only; covariates were scored based on reference values for categorical predictors or population mean for continuous covariates.
Mediation analysis
Higher levels of TRAP-NOx were also associated with prevalent T2DM (OR = 1.62 for the highest versus lower tertiles, 95% CI = 1.27, 2.05; OR = 1.24 per IQR, 95% CI = 1.11, 1.38; Table 3). And prevalent T2DM was also strongly related to incident dementia/CIND (HR = 1.94, 95% CI = 1.42, 2.66; Table 3), with similar results observed for time to dementia (HR = 1.93, 95% CI = 1.30, 2.87) and CIND only (HR = 2.24, 95% CI = 1.36, 3.71), results which have been described in more detail previously [38].
Table 3.
Relationship between TRAP (NOx) and type 2 diabetes and type 2 diabetes and incident cognitive outcomes.
| Outcome | Exposure Category | All SALSA (n=1564) |
Urban Only (n=1359) |
||||
|---|---|---|---|---|---|---|---|
| Outcome |
OR/HR |
Outcome | OR/HR | ||||
| No (%) | Yes (%) | (95% CI) | No (%) | Yes (%) | (95% CI) | ||
| Type 2 diabetes (T2DM) | NOx T3 (yes/no) | ||||||
| ≤ 2.68 ppb | 727 (68.3) | 307 (61.4) | 1.00 (ref) | 602 (64.5) | 273 (55.6) | 1.00 (ref) | |
| >2.68 ppb | 337 (31.7) | 193 (38.6) | 1.62 (1.27, 2.05) | 331 (35.5) | 189 (44.4) | 1.63 (1.27, 2.09) | |
| per IQR | 1.24 (1.11, 1.38) | 1.23 (1.10, 1.37) | |||||
| Time to incident dementia | Prevalent T2DM | ||||||
| No | 1009 (68.9) | 55 (55.0) | 1.00 (ref) | 886 (69.6) | 47 (54.7) | 1.00 (ref) | |
| Yes | 455 (31.0) | 45 (45.0) | 1.93 (1.30, 2.87) | 387 (30.4) | 39 (45.4) | 2.10 (1.37, 3.22) | |
| Time to incident CIND | Prevalent T2DM | ||||||
| No | 978 (69.4) | 37 (55.2) | 1.00 (ref) | 860 (70.0) | 32 (55.2) | 1.00 (ref) | |
| Yes | 432 (30.6) | 30 (44.8) | 2.24 (1.36, 3.71) | 368 (30.0) | 26 (44.8) | 2.36 (1.43, 3.91) | |
| Time to incident dementia/ CIND | Prevalent T2DM | ||||||
| No | 978 (69.4) | 86 (55.8) | 1.00 (ref) | 860 (70.0) | 73 (55.7) | 1.00 (ref) | |
| Yes | 432 (30.6) | 68 (44.2) | 1.94 (1.42, 2.66) | 368 (30.0) | 58 (44.3) | 2.09 (1.51, 2.91) | |
Abbreviations: CIND=Cognitive Impairment, non-dementia; T3=tertile 3 (TRAP NOx dichotomized at >T3); IQR=interquartile range (2.31 ppb) Based on logistic regression with census-tract clustered standard errors.
Models control for baseline 3MSE score, baseline age, sex, years of education, primary language spoken, occupation for most of life (manual, non-manual, or other), NSES, cigarette smoking, a county indicator, and a rural/urban residential neighborhood indicator (excluded for urban only analysis).
Using mediation analysis, we decomposed the total effect (TE) into the direct effect (DE) and indirect effect (IE) mediated through T2DM, and quantified the proportion mediated (Table 4). Across the cognitive outcomes (time to dementia, CIND, dementia/CIND), between 18% and 27% of the TE of TRAP-NOx was estimated to be mediated through T2DM (IE), while 82% to 73% was estimated to act through other pathways (DE). For example, for time to dementia/CIND among those who lived in urban populations, the TE of TRAP-NOx was estimated at HR = 1.59 (95% CI = 1.10, 2.26), meaning across all pathways, the total association of TRAP-NOx was estimated at HR = 1.59. In decomposing this total effect, looking at effects through T2DM (IE) specifically versus all other pathways (DE), we estimate the DE of TRAP-NOx at HR = 1.46 (95% CI = 1.00, 2.09) and IE at HR = 1.09 (95% CI = 1.04, 1.17). Indicating that 18% of the TE of TRAP-NOx on time to dementia/CIND was mediated through T2DM. Similar associations were observed for time to dementia. For CIND, the magnitude of the TE of TRAP-NOx was smaller and the confidence interval contains the null value (HR = 1.42; 95% CI = 0.79, 2.47). The IE mediated through T2DM, however, was similar in size (HR = 1.08, 95% CI = 1.02, 1.19), thus indicating a higher proportion (23%) was mediated through this pathway.
Table 4.
Mediation analysis. Total, direct, and indirect effects of TRAP (NOx) through type 2 diabetes on time to incident cognitive outcomes and proportion of total effect mediated through T2DM.
| All SALSA (n=1387)* |
Urban Only (n=1201) |
|||
|---|---|---|---|---|
| Associations | HR (95% CI) | Proportion Mediated | HR (95% CI) | Proportion Mediated |
| Total Effect: TRAP (NOx) (>2.68 ppb (T3) versus ≤ 2.68 ppb (ref)) → Dementia | 1.54 (0.96, 2.70) | 18% | 1.67 (1.01, 2.87) | 18% |
| Direct Effect: TRAP (NOx) → (M) → Dementia | 1.43 (0.88, 2.48) | 1.52 (0.92, 2.61) | ||
| Indirect Effect: TRAP (NOx) → T2DM → Dementia | 1.08 (1.03, 1.19) | 1.10 (1.03, 1.19) | ||
| Total Effect: TRAP (NOx) → CIND | 1.30 (0.71, 2.27) | 27% | 1.42 (0.79, 2.47) | 23% |
| Direct Effect: TRAP (NOx) → (M) → CIND | 1.21 (0.67, 2.08) | 1.31 (0.72, 2.25) | ||
| Indirect Effect: TRAP (NOx) → T2DM → CIND | 1.07 (1.01, 1.17) | 1.08 (1.02, 1.19) | ||
| Total Effect: TRAP (NOx) → Dementia/CIND | 1.40 (0.98, 2.07) | 20% | 1.59 (1.10, 2.26) | 18% |
| Direct Effect: TRAP (NOx) → (M) → Dementia/CIND | 1.32 (0.90, 1.92) | 1.46 (1.00, 2.09) | ||
| Indirect Effect: TRAP (NOx) → T2DM → Dementia/CIND | 1.06 (1.03, 1.14) | 1.09 (1.04, 1.17) | ||
Abbreviations: CIND=Cognitive Impairment, non-dementia; T3=tertile 3 (TRAP-NOx dichotomized at >T3); IQR=interquartile range (2.31 ppb) (M) = complete set of non-modeled mediators; i.e. all pathways not going through designated mediator Models control for baseline 3MSE score, baseline age, sex, years of education, primary language spoken, occupation for most of life (manual, non-manual, or other), NSES, IL-6 inflammation marker, cigarette smoking, a county indicator, and a rural/urban residential neighborhood indicator (excluded for urban only analysis)
Based on Cox proportional hazards models, time to event. Mediated proportion=IE/TE. 95% CI based on bootstrapping that accounts for clustering within census tracts (5,000 resamples; 2.5 and 97.5 percentiles).
This analysis differs from Table 2, in that we included IL-6 as a potential mediator-outcome confounder. As a result, 160 participants that did not have this information were excluded from the population used to calculate the estimates presented in this table. Thus, this slightly changes the sample size and model, and the total association therefore differs somewhat between Table 2 and Table 4.
In order to assess whether loss to follow-up was informative in our study, which may have resulted in potential selection bias, we also assessed whether our exposures and covariates were 1) related to censoring prior to the study end and 2) assessed how estimates changed when we included an IPCW. During the study period, 8 to 22% of the participants were lost and approximately 5% of the participants died after each exam (Supplementary Figure 1). In modeling loss to follow-up prior to exam 5, baseline cognitive function was predictive of loss, indicating those with better performance on the baseline 3MSE were less likely to drop out (OR per 1 point = 0.97, 95% CI = 0.96, 0.98). TRAP-NOx per IQR was also suggestively associated with loss (OR = 1.08 per IQR, 95% CI = 0.96, 1.21), but less so the dichotomized measure. Suggesting loss to follow-up may be informative and given the direction of associations (worse cognitive function and higher TRAP-NOx are both positively associated with loss), the Cox model results may be biased toward the null. To quantify this, we included the IPCW and modeled the time to event associations in the uncensored population only. These results are displayed in Supplementary Table 4. The findings were generally similar, but estimates are of somewhat greater magnitude, suggesting as expected loss to follow-up may have biased results toward the null. For example, for time to dementia/CIND, without including the IPCW, we observed a HR = 1.45 for higher TRAP-NOx (95% CI = 1.07, 1.96; Table 2), with the IPCW, we observed a HR = 1.54 (95% CI = 1.08, 2.19; Supplementary Table 4).
DISCUSSION
In this prospective cohort study of older, community-dwelling Mexican Americans from the Sacramento Valley (CA, USA), exposure to higher residential levels of local traffic-related air pollution was strongly associated with incident dementia, and somewhat less strongly with CIND. Applying a mediation analysis approach, we further observed that approximately 20% of the estimated effect of local TRAP-NOx on dementia or CIND was mediated through T2DM, suggesting that metabolic dysfunction is one of the pathways through which traffic-related air pollution exposure influences cognitive impairment and dementia among the elderly.
Only a handful of cohort studies previously investigated whether air pollution affects cognitive decline or dementia incidence (for review, see [45]). Two large cohorts, with long-term prospective follow-up and in-person clinical evaluations, the Betula study in Northern Sweden and the Women’s Health Initiative Memory Study (WHIMS), both reported that residential air pollution increased incident dementia risk (NOx (HR = 1.43, 95% CI = 1.00, 2.05) and PM2.5 (HR = 1.92, 95% CI = 1.32, 2.80), respectively) [4, 5]. The US based Nurses’ Health Study cognitive cohort, Normative Aging Cohort, and the REGARDS cohort found higher modeled ambient black carbon or PM at participants’ residence to be associated with cognitive decline [3, 46, 47]. Findings from an older cohort of Puerto Ricans in the Boston area provide support for an association between PM and cognitive decline in a Hispanic population [7]. The Whitehall II cohort from the greater London Area (UK), however, found only suggestive associations between a five-year decline on a standardized memory test and PM exposures [48]. In our elderly, Latino cohort we observe similar magnitude associations between local, traffic-related air pollution exposures and the cognitive outcomes reported in the Betula and WHIMS cohorts, both of which also assess incident dementia.
Furthermore, our findings not only confirm previous reports that traffic-related air pollution increases the risk of dementia/CIND among a Hispanic population, but we also estimated that approximately 20% of the effect of TRAP-NOx on dementia/CIND is mediated through T2DM. This observation suggests that a meaningful pathway through which TRAP-NOx acts on dementia/CIND is through metabolic function, with TRAP-NOx first potentially influencing metabolic dysfunction which in turn influences cognitive function. Several lines of research point toward mechanisms through which air pollution may impact brain health, broadly including chronic brain inflammation, microglia activation, and white matter abnormalities [49]. Specific to dementia, both in experimental and in postmortem studies, air pollution has been associated with amyloid-β plaques and hyperphosphorylated tau [50–52]. Another important mechanism is metabolic dysfunction, through which T2DM is also related to cognitive function, including systemic insulin resistance and vascular brain injury related to insulin resistance, and disruption of the normal action of insulin in the brain of T2DM patients [53]. Evidence for the involvement of air pollution in these pathways is increasing [54]. Air-pollution exposure has been shown to alter endothelial function [55, 56], which in turn precedes changes in insulin resistance, and has been implicated in reduced peripheral glucose uptake [57]. In fact, experimentally, PM2.5 exposure produced similar or even greater changes in insulin resistance measures than high-fat diets [58–60]. Tumor necrosis factor (TNF)-α, interleukin-6 (IL-6), resistin, and leptin levels were elevated following PM2.5 exposure, keeping with a proinflammatory insulin-resistant state [58–60]. Studies have also implicated air pollution exposure in additional pathways, including mitochondrial dysfunction and brown adipose tissue alterations, and hepatic insulin resistance and endoplasmic reticulum stress [54]. Taken together with our results, this research indicates that ambient air toxicants may not only have direct neuropathological effects, but also have indirect effects through diabetes and metabolic dysfunction affected by air pollution.
Quantifying the indirect pathways related to air pollution exposure in population studies may help elucidate the pathogenic mechanisms of exposure, as described above, in humans at relevant exposure levels. But more importantly, such knowledge may also help inform on preventative measures. Modification of proximal individual-level T2DM risk factors to improve brain health may be less successful should causal or potentiating upstream environmental exposures remain in place. Integrated measures that address both ambient pollution and lifestyle factors may have considerable implications for prevention of both outcomes, particularly in high-risk populations [49, 61, 62]. The majority of previous epidemiologic studies have been conducted in non-Hispanic white populations, where both the T2DM and dementia burden are lower than among Mexican Americans [12]. Our research among Mexican Americans provides important insights about the interrelatedness of air pollution and incident dementia and CIND with diabetes in a population with a high T2DM prevalence.
Furthermore, numerous studies have shown that low SES neighborhoods are affected by a disproportionate amount of air pollution exposure. For the SALSA cohort, we also observed that TRAP-NOx decreased with increasing neighborhood SES. Previously, lower neighborhood SES was associated with poorer cognitive scores in SALSA [10]. Education was estimated to explain about 20% of the within-neighborhood variance and 40% of the between-neighborhood variance for slopes of cognitive decline [10]. However, substantial variance in rates of cognitive decline remained unexplained, indicating that other risk factors including environmental exposures that aggregate in neighborhoods may also contribute to these outcomes.
The mediation analysis allowed us to quantify estimated indirect and direct effects. Operating under the counterfactual framework, the analysis assumes no uncontrolled confounding. We included a variety of individual demographic and SES factors, as well as a neighborhood SES score and census tract clustering. Still, this assumption is not testable in observational studies, and other confounding factors may exist. Furthermore, mediation analysis may be biased by the presence of mediator-outcome confounders that are affected by exposure. For instance, we hypothesized that inflammation may be a pathway through which TRAP-NOx acts on dementia/CIND. Inflammation is also associated with T2DM, thus may act as a mediator between TRAP-NOx and dementia/CIND, but a confounder of the T2DM and dementia/CIND association. We controlled for IL-6 levels at baseline to account for this, but other such confounders may also exist. Additionally, we mainly relied on prevalent T2DM in our analyses, and only observed suggestive associations between NOx and incident T2DM likely because exclusion of a third of the SALSA population due to prevalent T2DM limited our statistical power. Thus, we present this secondary mediation aim as first epidemiologic evidence linking air pollution, diabetes, and subsequent dementia in a population-based study, and hope other cohorts with longer follow-up for incident diabetes and incident dementia further assess these pathways to provide replication.
Neuropathological processes relevant to cognitive decline and dementia may begin 20 or more years prior to the onset of clinical symptoms [63]. Thus, exposures early in life and midlife are likely quite relevant for the development of cognitive decline and dementia later in life. We were limited in our exposure assessment to residential locations at baseline. However, since the relative spatial ranking of air pollution exposure from consistent sources (such as traffic) are likely steady over extended periods of time, it is fair to presume that these exposures at enrolment are adequate surrogates for past, long-term spatial contrasts. Also, residential mobility in the decade prior to baseline was quite low among SALSA participants. On average participants reported living at their baseline residence for 22 years with 75% of participants having not moved within 10 years, although 5% had lived at their baseline address for 2 years or less. Additionally, some changes in traffic density or major roadway construction may have influenced exposure patterns over the study period. It should also be emphasized that we modeled air pollution exposure due to local traffic-sources only using a comprehensive traffic database and a well-established dispersion model, thus, only changes in traffic pollution within 1500 meters of the homes would be relevant here. This is particularly relevant for the Sacramento region, as according to CARB, 85% of the area’s nitrogen oxides are from cars and trucks [64], indicating our local traffic-based air pollution assessment is generally appropriate for the region.
In terms of our model, a modified-CALINE4, numerous studies have verified estimates with field studies and measurements [34, 35], including our own internal verifications (Wu, J). Overall, the verifications have indicated that the CALINE4 model adequately estimates the measured concentration, but of course, there is likely some misclassification in our estimates from both modeling and other sources not included in CALINE4 models. Wind directional shifts have been determined as a significant factor for misclassification, and systematic overpredicting of estimates during very low (<1 m/s) parallel to the road wind conditions have been reported [34]. Thus, there is likely some exposure misclassification of our estimates; however, we expect this to be non-differential with respect to cognitive outcomes.
Furthermore, some discussion of generalizability, both spatially and temporally, is warranted. Air pollution in the Sacramento region has historically been relatively poor (in the top 10 most polluted cities in the US). There are geographical reasons along with sources of pollution; mountain ranges bordering the Central Valley limit dispersion and trap pollutants in the Valley. But since the study began (1998), strides have been made that improved air quality even as the population has grown. Overall, population and traffic density in the region has significantly increased. Between 2000–2016, the population grew by 25% and vehicle miles traveled increased by 36% [64]. However, there have been notable reductions in air pollution and NOx, primarily due to CA state regulations and air pollution control programs resulting in emission reductions. Regulations include requiring filters to be installed on diesel trucks and the use of new, less polluting diesel truck fleets as well as cleaner fuel regulations. During the same time period (2000–2016), NOx emissions over the Sacramento region were reduced by 61%, almost all from cars and trucks [64]. And the number of days the region exceeded the standard was reduced by 42%, despite growth in the region’s population and traffic [64].
In terms of relating this to our study findings, emission reductions from vehicles will mean less overall traffic-related air pollution in the region, which will no doubt lead to public health benefits. However, our findings remain quite relevant and likely generalizable, as we observed a meaningful increase in risk for dementia from low TRAP-NOx levels to mid-levels of exposure (see spline graphs), exposure levels that would still be present today. Thus the pollution level of mid-range exposure profiles from 1998/1999 may represent the pollution level of higher-range profiles today, which is a public health and environmental policy win. In addition, although air quality, especially traffic-related pollution has improved substantially in our study region, other areas worldwide (e.g., China, India, or locations with primarily Hispanic populations, such as Mexico, directly relevant to SALSA) still have high pollution levels due to rapid urbanization. Our results will have important and broad implications for assessing the adverse health impact of air pollution.
Our study has several strengths, including relying on a prospective community-based cohort of understudied Hispanics with a high burden of T2DM and regular follow-up visits over a 10-year period. Exams were conducted in person with home visits, and most participants were able to be seen for at least one follow-up exam. This allowed for repeated in-person neurologic and cognitive screens, clinical diagnoses of dementia and CIND, and measurements of fasting glucose that supplemented self-reported medical histories and medication use. However, loss to follow-up prior to study completion was related to cognitive performance at baseline and suggestively also to TRAP-NOx exposure. This informative loss to-follow-up likely led to an underestimation of the true TRAP-NOx relationship, due to both associations influencing loss in a positive direction. This was demonstrated in our IPCW analysis, where the effect estimates for TRAP-NOx were of greater magnitude after accounting for selection bias.
Conclusions
In the coming decades, numerous countries will face the challenges of a growing elderly population, particularly with regard to the personal, economic, and social burden of caring for those with dementia [65]. Actions that would result in reducing upstream dementia-related risk factors may mitigate this burden. Ambient air pollution exposures are long-term and affect large populations. While these exposures may yield relatively modest risk increases, they nevertheless result in large numbers of cases. Given the magnitude of exposure and broad range of health impacts, preventive actions targeting them provide great potential for prevention.
Our results suggest that exposure to local traffic-related air pollution (TRAP-NOx) is associated with incident dementia and cognitive impairment, non-dementia. Beyond this, we estimated 20% of the effect of TRAP-NOx on dementia/CIND to be mediated through T2DM, indicating an important indirect pathway through metabolic dysfunction. This research draws important connections between ambient air pollution and brain health via direct damage as well as indirect pathways related to diabetes and metabolic dysfunction.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Environmental Health Science (grant numbers F32ES028087 and R01ES023451), National Institute on Aging (grants AG012975, AG033751, and R00AG053410), National Institute of Diabetes, Digestive, and Kidney Diseases (DK060753).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0320r1).
Footnotes
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-200320.
REFERENCES
- [1].Brook RD, Rajagopalan S, Pope CAA, Brook JR, Bhatnagar A, Diez-Roux A V, Holguin F, Hong Y, Luepker R V, Mittleman MA, Peters A, Siscovick D, Smith SC, Whitsel L, Kaufman JD, American Heart Association Council on Epidemiology and Prevention, Council on Epidemiology and Prevention, Council on the Kidney in Cardivascualr Disease, Council on Nutrition, Physical Activity and Metabolism (2010) Particulate matter air pollution and cardiovascular disease. Circulation 121, 2331–2378. [DOI] [PubMed] [Google Scholar]
- [2].Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Kunzli N, Schikowski T, Probst-Hensch NM (2015) Association between ambient air pollution and diabetes mellitus in Europe and North America: Systematic review and meta-analysis. Env Heal Perspect 123, 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F (2012) Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med 172, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Oudin A, Forsberg B, Adolfsson AN, Lind N, Modig L, Nordin M, Nordin S, Adolfsson R, Nilsson LG (2016) Traffic-related air pollution and dementia incidence in Northern Sweden: A longitudinal study. Environ Health Perspect 124, 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cacciottolo M, Wang X, Driscoll I, Woodward N, Saffari A, Reyes J, Serre ML, Vizuete W, Sioutas C, Morgan TE, Gatz M, Chui HC, Shumaker SA, Resnick SM, Espeland MA, Finch CE, Chen JC (2017) Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry 7, e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Paul KC, Jerrett M, Ritz B (2018) Type 2 diabetes mellitus and Alzheimer’s disease: Overlapping biologic mechanisms and environmental risk factors. Curr Environ Health Rep 5, 44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wurth R, Kioumourtzoglou MA, Tucker KL, Griffith J, Manjourides J, Suh H (2018) Fine particle sources and cognitive function in an older Puerto Rican cohort in greater Boston. Environ Epidemiol 2, e022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Power MC, Lamichhane AP, Liao D, Xu X, Jack CR, Gottesman RF, Mosley T, Stewart JD, Yanosky JD, Whitsel EA (2018) The association of long-term exposure to particulate matter air pollution with brain MRI findings: The ARIC study. Environ Health Perspect 126, 027009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT (2016) Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther 8, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hazzouri AZ Al, Haan MN, Osypuk T, Abdou C, Hinton, Aiello AE (2011) Neighborhood socioeconomic context and cognitive decline among older mexican Americans: Results from the sacramento area latino study on aging. Am J Epidemiol 174, 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Glymour MM, Manly JJ (2008) Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev 18, 223–254. [DOI] [PubMed] [Google Scholar]
- [12].Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA (2016) Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 12, 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kravitz-Wirtz N, Crowder K, Hajat A, Sass V (2016) The long-term dynamics of racial/ethnic inequality in neighborhood air pollution exposure, 1990–2009. Du Bois Rev 13, 237–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jones MR, Diez-Roux AV, Hajat A, Kershaw KN, O’Neill MS, Guallar E, Post WS, Kaufman JD, Navas-Acien A (2014) Race/ethnicity, residential segregation, and exposure to ambient air pollution: The multi-ethnic study of atherosclerosis (MESA). Am J Public Health, 104, 2130–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vagelatos NT, Eslick GD (2013) Type 2 diabetes as a risk factor for Alzheimer’s disease: The confounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev 35, 152–160. [DOI] [PubMed] [Google Scholar]
- [16].Han W, Li C (2010) Linking type 2 diabetes and Alzheimer’s disease. Proc Natl Acad Sci U S A 107, 6557–6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Alzheimer’s Association (2018) 2018 Alzheimer’s disease facts and figures. Alzheimers Dement 14, 367–429. [Google Scholar]
- [18].Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA (2009) The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 66, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schneider JA, Arvanitakis Z, Bang W, Bennett DA (2007) Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69, 2197–2204. [DOI] [PubMed] [Google Scholar]
- [20].Kandimalla R, Thirumala V, Reddy PH (2017) Is Alzheimer’s disease a Type 3 diabetes? A critical appraisal. Biochim Biophys Acta Mol Basis Dis 1863, 1078–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vella RE, Pillon NJ, Zarrouki B, Croze ML, Koppe L, Guichardant M, Pesenti S, Chauvin MA, Rieusset J, Géloën A, Soulage CO (2015) Ozone exposure triggers insulin resistance through muscle c-Jun N-terminal kinase activation. Diabetes 64, 1011–1024. [DOI] [PubMed] [Google Scholar]
- [22].Bass V, Gordon CJ, Jarema KA, MacPhail RC, Cascio WE, Phillips PM, Ledbetter AD, Schladweiler MC, Andrews D, Miller D, Doerfler DL, Kodavanti UP (2013) Ozone induces glucose intolerance and systemic metabolic effects in young and aged brown Norway rats. Toxicol Appl Pharmacol 273, 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S (2003) Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A 100, 4162–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Eckman EA, Eckman CB (2005) Abeta-degrading enzymes: Modulators of Alzheimer’s disease pathogenesis and targets for therapeutic intervention. Biochem Soc Trans 33, 1101–1105. [DOI] [PubMed] [Google Scholar]
- [25].de la Monte SM, Wands JR (2008) Alzheimer’s disease is type 3 diabetes – evidence reviewed. J Diabetes Sci Technol 2, 1101–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ (2003) Prevalence of dementia in older Latinos: The influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc 51, 169–177. [DOI] [PubMed] [Google Scholar]
- [27].California Department of Transportation, Federal Highway Administration, Benson PE (1989) CALINE4 – a dispersion model for predicting air pollutant concentrations near roadways. FHWA-CA-TL-84–15 Final Rpt [Google Scholar]
- [28].Wu J, Houston D, Lurmann F, Ong P, Winer A (2009) Exposure of PM2.5 and EC from diesel and gasoline vehicles in communities near the ports of Los Angeles and Long Beach, California. Atmos Environ 43, 1962–1971. [Google Scholar]
- [29].Wu J, Laurent O, Li L, Hu J, Kleeman M (2016) Adverse reproductive health outcomes and exposure to gaseous and particulate-matter air pollution in pregnant women. Res Rep Health Eff Inst 188, 1–58. [PMC free article] [PubMed] [Google Scholar]
- [30].Zhu Y, Hinds WC, Kim S, Shen S, Sioutas C (2002) Study of ultrafine particles near a major highway with heavy-duty diesel traffic. Atmos Environ 36, 4323–4335. [Google Scholar]
- [31].Hu S, Fruin S, Kozawa K, Mara S, Paulson SE, Winer AM (2009) A wide area of air pollutant impact downwind of a freeway during pre-sunrise hours. Atmos Environ (1994) 43, 2541–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].California Air Resources Board: Sacramento CA (2015) California Air Resources Board (CARB), Air Quality and Meteorological Information System. [Google Scholar]
- [33].California Environmental Protection Agency (EPA) Air ResourcesBoard.EMFAC2011–TechnicalDocumentation, California Air Resources Board, Sacramento, CA. [Google Scholar]
- [34].Benson PE (1988) Development and verification of the California Line Source Dispersion Model. Transportation Research Board. [Google Scholar]
- [35].Chen H, Bai S, Eisinger D, Niemeier D, Claggett M (2009) Predicting near-road PM 2.5 concentrations: A comparative assessment of CALINE4, CAL3QHC and AERMOD. Transp Res Rec 2123, 26–37. [Google Scholar]
- [36].Mungas D, Reed BR, Farias ST, Decarli C (2005) Criterion-referenced validity of a neuropsychological test battery: Equivalent performance in elderly Hispanics and Non-Hispanic Whites. J Int Neuropsychol Soc 11, 620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. American Psychiatric Association, Washington, DC. [Google Scholar]
- [38].Mayeda ER, Haan MN, Yaffe K, Kanaya AM, Neuhaus J (2015) Does type 2 diabetes increase rate of cognitive decline in older mexican americans? Alzheimer Dis Assoc Disord 29, 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yost K, Perkins C, Cohen R, Morris C, Wright W (2001) Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 12, 703–711. [DOI] [PubMed] [Google Scholar]
- [40].Hernán M, Robins J (2017) Causal Inference. Chapman & Hall/CRC, Boca Raton, FL. [Google Scholar]
- [41].Lange T, Vansteelandt S, Bekaert M (2012) A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol 176, 190–195. [DOI] [PubMed] [Google Scholar]
- [42].Pearl J (2011) Causality: Models, reasoning, and inference, second edition Cambridge University Press. [Google Scholar]
- [43].Rochon J, Du Bois A, Lange T (2014) Mediation analysis of the relationship between institutional research activity and patient survival. BMC Med Res Methodol 14, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].R Foundation for Statistical Computing (2018) R: A language and environment for statistical computing. [Google Scholar]
- [45].Paul KC, Haan M, Mayeda ER, Ritz BR (2019) Ambient air pollution, noise, and late-life cognitive decline and dementia risk. Annu Rev Public Health 1, 203–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Loop MS, Kent ST, Al-Hamdan MZ, Crosson WL, Estes SM, Estes MG, Quattrochi DA, Hemmings SN, Wadley VG, McClure LA (2013) Fine particulate matter and incident cognitive impairment in the reasons for geographic and racial differences in stroke (REGARDS) cohort. PLoS One 8, e75001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Power MC, Weisskopf MG, Alexeeff SE, Coull BA, Avron S, Schwartz J (2011) Traffic-related air pollution and cognitive function in a cohort of older men. Environ Health Perspect 119, 682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tonne C, Elbaz A, Beevers S, Singh-Manoux A (2014) Traffic-related air pollution in relation to cognitive function in older adults. Epidemiology 25, 674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen JC, Cory-Slechta DA, Costa D, Diaz-Sanchez D, Dorman DC, Gold DR, Gray K, Jeng HA, Kaufman JD, Kleinman MT, Kirshner A, Lawler C, Miller DS, Nadadur SS, Ritz B, Semmens EO, Tonelli LH, Veronesi B, Wright RO, Wright RJ (2012) The outdoor air pollution and brain health workshop. Neurotoxicology 33, 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, Villarreal-Calderón R, Osnaya N, Stone I, García R, Brooks DM, González-Maciel A, Reynoso-Robles R, Delgado-Chávez R, Reed W´ (2008) Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid β−42 and α-synuclein in children and young adult. Toxicol Pathol 36, 289–310. [DOI] [PubMed] [Google Scholar]
- [51].Calderón-Garcidueas L, Kavanaugh M, Block M, D’Angiulli A, Delgado-Chávez R, Torres-Jardón R, González-Maciel A, Reynoso-Robles R, Osnaya N, Villarreal-Calderon R, Guo R, Hua Z, Zhu H, Perry G, Diaz P (2012) Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J Alzheimers Dis 28, 93–107. [DOI] [PubMed] [Google Scholar]
- [52].Levesque S, Taetzsch T, Lull ME, Kodavanti U, Stadler K, Wagner A, Johnson JA, Duke L, Kodavanti P, Surace MJ, Block ML (2011) Diesel exhaust activates and primes microglia: Air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ Health Perspect 119, 1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Cholerton B, Baker LD, Montine TJ, Craft S (2016) Type 2 diabetes, cognition, and dementia in older adults: Toward a precision health approach. Diabetes Spectr 29, 210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rajagopalan S, Brook RD (2012) Air pollution and type 2 diabetes: Mechanistic insights. Diabetes 61, 3037–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mills NL, Törnqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE (2005) Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 112, 3930–3936. [DOI] [PubMed] [Google Scholar]
- [56].Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JGS, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S (2005) Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. J Am Med Assoc 294, 3003–3010. [DOI] [PubMed] [Google Scholar]
- [57].Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G (1995) Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest 96, 786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Laing S, Wang GH, Briazova T, Zhang CB, Wang AX, Zheng Z, Gow A, Chen AF, Rajagopalan S, Chen LC, Sun QH, Zhang KZ (2010) Airborne particulate matter selectively activates endoplasmic reticulum stress response in the lung and liver tissues. Am J Physiol Physiol 299, C736-C749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Xu X, Yavar Z, Verdin M, Ying Z, Mihai G, Kampfrath T, Wang A, Zhong M, Lippmann M, Chen LC, Rajagopalan S, Sun Q (2010) Effect of early particulate air pollution exposure on obesity in mice: Role of p47phox. Arter Thromb Vasc Biol 30, 2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yan YH, Chou CC, Lee CT, Liu JY, Cheng TJ (2011) Enhanced insulin resistance in diet-induced obese rats exposed to fine particles by instillation. Inhal Toxicol 23, 507–519. [DOI] [PubMed] [Google Scholar]
- [61].O’Neill MS, Jerrett M, Kawachi I, Levy JI, Cohen AJ, Gouveia N, Wilkinson P, Fletcher T, Cifuentes L, Schwartz J, Bateson TF, Cann C, Dockery D, Gold D, Laden F, London S, Loomis D, Speizer F, Van den Eeden S, Zanobetti A (2003) Health, wealth, and air pollution: Advancing theory and methods. Environ Health Perspect 111, 1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].O’Neill MS, Kinney PL, Cohen AJ (2008) Environmental equity in air quality management: Local and international implications for human health and climate change. J Toxicol Environ Heal Part A 71, 570–577. [DOI] [PubMed] [Google Scholar]
- [63].Alzheimer’s Association (2017) 2017 Alzheimer’s disease facts and figures. Alzheimers Dement 13, 325–373. [Google Scholar]
- [64].CARB (2017) Sacramento region set to meet federal health-based air quality standard two years before deadline. California Air Resources Board, Sacramento, CA: https://ww2.arb.ca.gov/news/sacramento-region-set-meet-federal-health-based-air-quality-standard-two-years-deadline [Google Scholar]
- [65].United Nations (2002) Report of the Second World Assembly on Ageing: Madrid, 8–12 April 2002, United Nations Publications. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.