Table 3.
Practical use, in-vitro and in-vivo degree of antiviral activity, therapeutic & selectivity index, toxicity and clinical study profile of few selected marker phytocompounds derived from herbs.
| Compounds (PubChem ID) & structure | IC50/EC50 (in vitro degree of antiviral activity) [CC50: at which dose of the test compound kills 50% normal host cell SI=CC50/EC50 | In-vivo degree of antiviral activity in animal model | Toxicity | Clinical Trial/Stage of development | References |
|---|---|---|---|---|---|
Emodin (3220)
|
200 μM against SARS-CoV-1 by inhibiting interaction of Spike protein and Vero E6 cell | - | - | - | Ho et al., 2007 |
| EC50 against Human Influenza A is 4.25μg/ml, inhibit viral replication at CC50 of 182.95 μg/ml against MDCK cells. | - | Emodin is apparently non-toxic at low therapeutic dose in Preclinical Rat model due to extensive glucuronidation, however high doses may cause renal or hepatic toxicity. | Observational study conducted on human breast cancer without any confirmed result. |
Dai et al., 2017; Dong et al., 2016; https://clinicaltrials.gov/ct2/show/record/NCT01287468 |
|
| - | Emodin significantly increase the survival rate and promote recovery of HSV-infected mice; equivalent to acyclovir in vivo at 6.7 and 11.3 g/kg/day for 7 days. | - | - | Xiong et al., 2011 | |
|
Luteolin (5280445) 
|
EC50 is 10.6 μM against wild-type SARS-CoV with selectivity index of 14.62 | 100 mg/kg for 3 days of post DENV-2 (clinical isolates) infection in mice; reduce viremia 2-fold, compared to untreated control. | Quite safe in preclinical animal study with LD50 of 456 mg/kg. Used safely with high SI without any substantial toxicity (Yi et al., 2004) | Luteolin enrich Pomegranate juice have been shown good efficacy to ameliorate the C-reactive protein in inflammatory disorders at randomized controlled trial without any serious adverse effect luteolin enrich Açaí Palm Berry extract in Phase 2 for Intervention in COVID-19 Patients. |
Yi et al., 2004; Peng et al., 2017; Sahebkar et al., 2016 https://clinicaltrials.gov/ct2/show/NCT04404218 |
|
Nicotionamine (9882882) 
|
IC50 was found to be 84 nM for Nicotionamine to inhibit recombinant ACE2 activity of host cell. | - | Quite safe and non-toxic to normal mammalian cells at therapeutic dose without any apparent adverse effect. | A Double-Blind-Randomized, Placebo-controlled Adaptive Design Phase 2 Trial in Mild Cognitive Impairment due to Alzheimer's disease are conducted successfully. | Takahashi et al., 2015; https://clinicaltrials.gov/ct2/show/NCT03061474 |
|
Andrographolide 5318517 
|
EC50 was 22 µM against Dengue virus. EC50 against H9N2, H5N1, H1N1 influenza viral strain was 94.3, 121.7, 110.0 μM with CC50 3085 µM in MDCK cells. |
Out of 10, nine infected mice were survived and recovered from H1N1 influenza virus infection 500 mg/kg. | Does not produce AMES toxicity, Maximum tolerated dose for human is 0.128 log mg/kg/day. Does not inhibit hERG-I and hERG-II. (LD50 2.162 mol/kg). Chronic oral rat toxicity (LOAEL) was found to be 1 log mg/kg body weight/day. Does not produce hepatotoxicity or cause skin sensitivity. LD50 >4000 mg /kg in mice without any apparent toxicity. |
In China it is used clinically to treat pain, fever and inflammation in viral infection. Phase 1 study in HIV patients and healthy volunteer showed no toxicity on normal population. Phase 4 clinical trial was conducted for Acute Exacerbation of Chronic Bronchitis and Acute Tonsillitis. |
Sukanth et al., 2020; Panraksa et al., 2017; Chen et al., 2009; Chen et al., 2010; Calabrese et al., 2000; Enmozhi et al., 2020 https://clinicaltrials.gov/ct2/show/NCT03134443 https://clinicaltrials.gov/ct2/show/NCT03132610 |
|
Polyphenolic compounds: Ellagic acid (5281855)  Myricetin (5281672)  (+)-Catechin (9064) 
|
Dose-dependent inhibitory activity against MERS/SARS-CoV proteases with IC50 of 30.2 to 233.3 μM. | Ellagic acid, and Myricetin like polyphenols have potentials at 1.0 mg/kg as anti-influenza agents in vivo. | No pronounced toxicity was shown in animal model at 1.0 mg/kg | Catechin rich polyphenolic extract of Green tea recruited in Randomized human clinical trial for prevention of influenza infection through gargling and dietary consumption in young and adults successfully completed. |
Park et al., 2017; Park et al., 2013 ; https://clinicaltrials.gov/ct2/show/NCT01225770 https://clinicaltrials.gov/ct2/show/NCT01008020 |
|
Bromhexine (2442) 
|
IC50 0.75μM against TMPRSS2 receptor expression |
Induce expectorant activity significantly at 12 mg/kg for 3 days treatment in RAT model | Induce liver toxicity when given chloroquine/HCQ with liver damage in COVID-19. Exploratory results of the study support our proposal that bromhexine hydrochloride may have a good effect on the treatment of COVID-19. |
A clinical trial (registration number NCT04273763), carried out by WEPON Pharmaceutical Co. Ltd., is the first human-based preliminary exploratory randomized-controlled clinical study on treating COVID-19 with bromhexine hydrochloride tablets (BHT). | Roberto et al., 2020. Markus et al., 2020; Depfenhart et al., 2020 https://clinicaltrials.gov/ct2/show/NCT04273763 |
|
Baicalein (5281605) 
|
EC50 of Baicalein was 2.94 μM in SARS-CoV-2 clinical isolates on Vero E6 cells with CC50>200 μM | It significantly decrease viral load and mortality in HSV infection at 200 mg/kg; Als0 attenuates acute lung injury triggered by influenza A infection at 100 mg/kg in mouse model. | Single oral dose of 100–2800 mg of Baicalein were safe and well tolerated by healthy subjects (clinical trials registration number CTR20132944) | Baicalein has been registered for safety and efficacy evaluation in randomized, double-blind, placebo-controlled trial for influenza infection in Phase 2 Clinical trial. |
Su et al., 2020; Zhi et al.,2020; Luo et al., 2020 https://clinicaltrials.gov/ct2/show/NCT03830684 |
Celastrol (122724)
|
IC50 against nSARS-CoV 3CLpro is 10.3 μM. Celastrol inhibit replication of DENV-2 viral strain with IC50 of 0.12 ± 0.11 μM. |
It significantly attenuates the auto-immune disorder related inflammation in Trex1-deficient Wild type C57BL/6 mice at 1.0 mg/kg Celastrol given for 3 days without apparent toxicity Another study showed that Celastrol at 0.1 mg/kg defended 80% of the mice against life-threatening DENV-2 infection. | Higher doses of Celastrol, beyond therapeutic dose, shows infertility, QT prolongation, and hepatotoxicity | No clinical trial reported so far. |
Ryu et al, 2010b; Liu et al., 2020; Hou et al., 2020; Yu et al., 2017 |
|
Plant Lectins 2GTY (PDB ID) 
|
Griffithsin, a potential Lectin inhibited infectivity of MERS-CoV infection by more than 90% in cell Culture assay. |
It demonstrates remarkable activity with satisfactory survival rate against SARS-CoV-infected mice at 5 mg/kg intra nasal route followed by two daily doses at 2.5 mg/kg. H84T, a banana lectin confers more than 80% protection against broad spectrum influenza A viral infection at 0.1 mg/kg |
Griffithsin, glycoprotein-based lectin shows high therapeutic index without any considerable side effect. Banana lectin does not show any toxicity at normal mammalian cells at anti-viral doses |
A Randomized, Double-Blind Phase- 1 Safety and Pharmaco-kinetic Study of Q-Griffithsin Enema administered to prevent HIV-1 entry on Sero- negative adults was reported |
Millet et al., 2016, O'Keefe et al., 2010; Covés-Datson et al., 2020; https://clinicaltrials.gov/ct2/show/NCT04032717 |
|
Ursolic acid (64945) 
|
IC50 against Influenza virus type 1, and HIV was > 200 μg/ml and 0.3 µM respectively. Further, Ursolic acid inhibit HSV-1and HSV-2 at EC50 of 5.5 and 5.8 µg/ml at SI of 20 and 18.97 (Bag et al., 2012). |
N.D. | Parasite infected mice treated with Ursolic acid did not exhibit any alterations in biochemical parameters, support that this triterpene is non-toxic for animals. Considering the low or absent of toxicity of triterpenes for mice, as well as high Trypanocidal activity. | N.D. |
Kashyap et al., 2016 Bag et al., 2012 |
|
acetyl-11-keto-boswellic acid (11168203) 
|
IC50 against clinical isolates of Herpes simplex virus-1 was 12.1 μg/ml | N.D. | It is nontoxic at experimental therapeutic dose | It has been recruited for complementary therapy as along with Glycyrrhiza glabra extract for prevention of Covid-19. |
Goswami et al., 2018 https://clinicaltrials.gov/ct2/show/NCT04487964 |
|
Cepharanthine (10206) 
|
IC50 was 9.5 μg/ml against some Corona virus strains. | It reduces tumor growth and volume by inhibiting STAT3 expression at 20-mg/kg/day for 20 days in xenograft mice model of SaOS2 osteo-sarcoma. | It reduces the chemotherapy induced renal toxicity. While Endotoxin induced lethal toxicity can be bypassed by this compound. It is quite safe at its anti-tumor in-vivo doses. | N.D. |
Zhang et al., 2005; Bailly, 2019 |
Sylimarin (5213)
|
Silymarin showed anti-viral activity against influenza virus A/PR/8/34 with 98% protection at 100 μg/ml with IC50 of 11.12 μg/ml. |
Silybin, active constituent of Silymarin, revealed significant reduction of HCV load within 16 days of treatment at 20 mg/kg by reducing baseline mean value of viral load in Phase 3 clinical trials without any untoward effect. | It is completely safe and did not show any apparent toxicity at its anti-viral dose. | A randomized placebo-controlled trial in Phase 3 is carrying out to assess the clinical outcome in COVID-19 Pneumonia by silymarin due to its ability to inhibit p38 MAPK pathway and its antiviral, anti-inflammatory and anti-oxidant potential. |
Liu et al., 2019; Rendina et al., 2014; Song and Choi, 2011 https://clinicaltrials.gov/ct2/show/NCT04394208 |
|
Epigallocatechin 3-gallate (EGCG) (65064) 
|
Attenuate the binding activity of SARS-CoV Nucleocapsid protein with QDs-conjugated RNA oligonucleotide on immobilized protein chip at concentrations ≥0.005µg/ml, IC50: 0.05µg/ml for activity against SARS. | Reduced the core hepatitis B viral antigen expression and replication at 25 mg/kg/day for 5 days i.p. treatment. | Oral acute LD50 was 2170 mg/kg in Rat. High therapeutic dose (100 mg/kg/day) did not produce any adverse side effects, without changing body weight, genotoxicity, behavior or mortality of treated animals. |
Safety, toxicity, dosing, and antiviral effects of EGCG at 200 mg in capsule form (orally twice daily at three different doses) in HIV-1-infected Patient was carried out successfully through Randomized interventional Clinical Trial. |
Roh, 2012; Wang et al, 2020; Ferreira et al, 2012; Isbrucker et al, 2006; https://clinicaltrials.gov/ct2/show/NCT01433289 |
|
Quercetin (5280343)  Rutin (5280805) 
|
IC50 against SARS-CoV-1 3CLPro and PLpro was 52.7±4.1 μM and 8.6±3.2 μM, respectively. IC50 of Rutin was >25 μM against Feline Coronavirus replication. |
Quercetin significantly reduced viral load of Rhinovirus infection (95.84%) at 0.2 mg/mouse for 4-day treatment without showing any toxicity. | Quercetin has no adverse effect at therapeutic dose and did not show any carcinogenicity or genotoxicity | Quercetin applied in Q-Trial in HCV (Q) patients, result not yet published. |
Park et al., 2017; McDonagh et al., 2014; Ganesan et al., 2012; Harwood et al., 2007 https://clinicaltrials.gov/ct2/show/NCT01438320 |
|
Glycyrrhizic acid (14982) 
|
At EC50 of 365 µM it inhibit SARS-CoV replication in Vero cells with Selectivity Index of > 65 (CC50 >24000 µM) |
Showed remarkable protection by 70% survival against 20 LD50 of influenza A2 at 10 mg/kg per day before infection and 1- and 4-days post infection. |
GL has no apparent adverse reaction or toxicity at lower doses; and have no genotoxicity. | Dietary Supplement: Licorice extract containing Glycyrrhizic acid has been used for complementary intervention of COVID-19 in interventional Clinical Trial. | Hoever et. al, 2005; Utsunomiya et. al,1997; Ming et al., 2013 https://clinicaltrials.gov/ct2/show/NCT04487964 |
RESVERATROL (RE) (445154)
|
RE inhibit nucleocapsid protein translation of MERS-CoV without any toxicity on Vero E6 cells at IC50 of 250 μM. |
RE significantly reduces levels of Rota virus antigens in the colon, jejunum, and ileum, compared to untreated mice at 4-10-day post infection with increasing rate of survival and decreasing diarrhea scores. It may lead to weight gain at 20 mg/kg orally. | Orally administered RE at 200 mg/kg/day in Rats and 600 mg/kg/day in Dogs for 90 days, did not show any side effect. But high dose of RE acts as pro-oxidant, thyroid disruptor and a goitrogen. In a phase II clinical trial on patients with refractory multiple myeloma, a daily dose of 5.0 g of RE showed nausea, diarrhea, fatigue, and renal toxicity. |
SARS-CoV-2 Viral Load and COVID-19 Disease Severity be Reduced by Resveratrol-assisted Zinc therapy at Phase 2 trial, proposed but not yet recruited. Dietary supplement of RE at 500 mg tablet for 30 days on Double-blind Cross-over Randomized Controlled Trial reduce the systemic inflammatory state & oxidants-antioxidants imbalance of tobacco users in Phase 3 study. |
Lin et al., 2017; Huang et al., 2020; Shaito et al., 2020; https://clinicaltrials.gov/ct2/show/NCT04542993; https://clinicaltrials.gov/ct2/show/NCT01492114 |
Cannabidiol (644019)
|
EC50 against Hepatitis C virus was 3.163±0.133 μM with selectivity index of 4.954 in normal mammalian cell. | TMEV-infected mice treated for 7 consecutive days with CBD (5 mg/kg) reduced the brain leucocyte infiltration and other inflammatory cytokine and chemokine expression; thereby increase the survival rate of the virus infected mice. Both in HIV and in post-Ebola syndrome Cannabidiol has been recommended as therapeutic agent to control immune activation at doses of 10–20 mg/kg/day and 1.7–10 mg/kg/day. |
CBD shows developmental toxicity, embryo-fetal mortality, central nervous system inhibition and neurotoxicity, hepatocellular injuries, sperm reduction, organ weight alterations, male reproductive system alterations, and hypotension, at doses higher than recommended for human. Intravenous LD50 of CBD on Rhesus monkey is 212 mg/kg |
syndrome, cannabidiol has been proposed as a therapeutic agent to control immune activation at doses of 10–20 mg kg−1 day−1 and 1.7–10 mg kg−1 day−1 (100 mg day−1titrating up to 600 mg day−1), respectively Cannabis is used as medicine for Prevention and Treatment of COVID-19, has been recruited for Non-randomized clinical trial in Phase 2 with 200000 participants. |
Lowe et. Al., 2017; Costiniuk et. al, 2020; Esposito et al., 2020; Mecha et al., 2013; Huestis et al.,2019 https://clinicaltrials.gov/ct2/show/NCT03944447 |
|
Tetrahydrocannabinol (THC) (2978) 
|
- | THC reduced the sign of ARDS in SEB induced severe acute respiratory syndrome mice at 20 and 10 mg /kg i.p. dose and suppressed the inflammatory cytokine storm. | Oral LD50 of THC in mice is 482 mg/kg and in Rat oral LD50 is 666 mg/kg. THC is not genotoxic, carcinogenic or causes remarkable immunosuppression during acute inflammation and not shown any apparent side effect or acute adverse reaction at therapeutic dose in mice. |
Double-Blind, Randomized, study with oral Dronabinol Versus Placebo was conducted in the treatment or prevention of Highly Active Antiretroviral Therapy (HAART)-related nausea and vomiting in phase 2. |
Mohammed et al., 2020; Chilakapati and Farris, 2014; https://clinicaltrials.gov/ct2/show/NCT00642499 |
Berberine (2353)
|
IC50 against mouse hepatitis virus was 2.0±0.5 μM with S.I. of 34.9. IC50 against influenza A H1N1 strain was: 0.44 μM |
It drastically reduced the viral load of H1N1 influenza infection in mice lungs at 7 days post infection treatment at 20 mg/kg. | Non-toxic and safe at therapeutic dose. | Effect of Berberine on Intestinal function and Inflammatory mediators in severe COVID-19 Patients was carried out, based on prospective randomized controlled clinical trial in Phase 4. |
Mani et al., 2020; Cecil et al., 2011; Yan et al., 2018; https://clinicaltrials.gov/ct2/show/NCT04479202 |
Sinigrin (23682211)
|
IC50 against cell-free (3CLpro.) and cell-based cleavage activity of SARS-CoV was 121 and 217 μM with CC50 >10,000 μM. | N.D. | Fetotoxicity, Non-carcinogenic and non-mutagenic at safe doses. Oral LD50 is 644 mg/kg in Rat. |
N.D. on viral disease | Lin et al., 2005; https://www.chemsrc.com/en/cas/3952-98-5_673009.html |
Hesperetin (72281)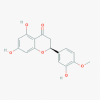
|
IC50 against cell-free (3CLpro.) and cell-based cleavage activity of SARS-CoV was 60 and 8.3 μM with CC50 -2718 μM. | Every two days at 5 mg/kg dose given to mice with cancer for 30 days, showed significant reduction of tumor without toxicity. | Does not induce any apparent toxicity within the therapeutic dose and no damage to human cell lines GES-1. | An Open-Label study to evaluate the effect of Elimune (2 capsules BID for 28 days; Hesperetin containing Biomarkers in Patients with Plaque Psoriasis. |
Lin et al., 2005; He et al., 2020 https://clinicaltrials.gov/ct2/show/NCT02251678 |
|
Allitridin (16315) 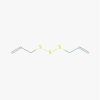
|
Inhibition of HCMV replication via suppression of viral IE gene transcription with IC50 of 20 μg/ml and selectivity index of 16.7 on human HEL cell. | Reduced MCMV DNA load and hepatic leision in Murine cytomegalovirus model at 0.14 and 0.42 mM/kg/day for 18 and 14 days. | Intravenous use of Allitridin is safe and relatively free of negative side-effects. LD50 in mice following intravenous injection was 110.9 mg/kg |
N.D. |
Zhen et al. 2006; Liu et al. 2004; Shen et al., 1996; Li et al., 2011 |
Alvocidib (Flavopiridol) [5287969]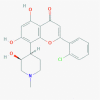
|
IC50 against Influenza A H7N9, pdm H1N1 and H3N2 Replication: 0.24, 0.59 and 0.70μM respectively, with selectivity index of >425, 170, and 142. EC50 against HIV replication: 8–15 nM. |
Flavopiridol (2.5 mg/kg every 12 h) for 20 days suppressed HIV-1 transcription in mouse kidney in HIV transgenic mouse model. | Flavopiridol treatment to both animals and humans at doses within the therapeutic range in vivo displays no apparent toxicity. | In cancer this molecule has been tested successfully in phase 3 for various type of lymphoma, leukemia; but in HIV infection it is not yet recruited. |
Perwitasari et al., 2015; Nelson et al., 2003; Sadaie et al., 2004 |
Ginsenoside Rg3(9918693)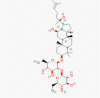
|
IC50 of 20(S)-Rg3 against MHV-68 viral replication: 10.82 ±1.56 μM | At 1.0 mg/mouse 55% improvement against infection of hemagglutinating virus of Japan (HVJ) at 11 days post-infection. | The no-observed-adverse-effect of Rg3 for dogs were considered to be 7.20 mg/kg/day in 26 days intramuscular injection after recovery from tumerosis. Body weight, food intake, ophthalmoscopy, electrocardio-gram, urinalysis, hematology, serum biochemistry, gross and histopathology findings were normal. | A Randomized, Double-blind, Placebo-controlled, Parallel-group Clinical study was done to evaluate the efficacy and safety of Ginsenoside, using Rg3 Capsule in Prevention of Postoperative recurrence of Hepatocellular Carcinoma. |
Kang et al., 2018; Liu et al., 2011 https://clinicaltrials.gov/ct2/show/NCT01717066 |
Lycorine (72378)
|
EC50 15.7 nM and CC50 value 14980.0 nM against SARS-CoV EC50 0.8 μM and CC50 of 25.1 μM against DENV. |
Protect the ZIKV infected AG6 mice mortality rate by 83%, 66 and 33% following the reduction of viral load and its replication at 10, 5.0 and 1.0 mg/kg body wt respectively. |
A potential candidate for cancer therapy and other viral infection with low toxicity. It also posses anti-oxidant, anti-toxic, and hepato-protective effect with safe therapeutic window. LD50 via i.p. in mice was 112.2±0.024 mg/kg. LD50: 344 mg/kg via gastric lavage injection in mice |
N.D. |
Li et al., 2005, Li et al., 2005; Cao et al., 2013, Cao et al., 2013 Wang et al., 2014; Chen et al., 2020; |
Vitexin (5280441)
|
IC50 against Parainfluenza type 3 virus was: 20.8μg/ml with CC50 333.3 μg/ml IC50 against Rota virus was: 129 μM |
Vitexin at 2 mg/kg i.v. successfully exert 55% protection against the middle cerebral apoptosis and ischemic stroke via suppressing proinflammatory cytokines (TNF-α and IL-6) and stimulating anti-inflammatory cytokine (IL-10) in Rat model. | It completely devoid of any adverse reaction and toxicity within the therapeutic window in animal model due to its cell protecting and anti-oxidant activity. | Not yet recruited in viral diseases or associated disorders |
Gandhi et al., 2016; Li et al., 2002; Jiang et al., 2018 |
|
Chlorogenic acid (1794427) 
|
IC50 against SARS-CoV-2 3CLpro was 39.48 ± 5.51 µM EC50 against oseltamivir sensitive and resistant strain of influenza virus: 39.42 & 58.34 μM respectively with CC50 against MDCK cells 364.30 μM |
Intravenous injection of 100 mg/kg/day exerted promising antiviral effect in mice, conferring 60% and 50% protection from death against H1N1 and H3N2 infection, reduce virus titers and effectively attenuate inflammation in the lungs, inhibiting neuraminidase. | LD50 in Rat (i.p.) at 3000 mg/kg. It did not produce any unwanted toxicity up to 4000 mg/kg in Rat. Neither Teratogenic, nor CNS toxic. |
Dietary supplement of Açaí Berry extract containing Chlorogenic acid (3 capsules of 520 mg for 30 days) was given in Phase 2 for alleviating inflammation of Covid-19 patients. |
Su et al., 2020; Ding et al., 2017; Chaube et al., 1976; https://clinicaltrials.gov/ct2/show/NCT04404218 |
Mangiferin (5281647)
|
IC50 against HIV was: 3.59 μg/ml and CC50 in MT-2 cells: 125 μg/ml EC50 against HIV-1KM018 isolate and HIV-1A17 resistant strain: 35.40 & 22.75 μM respectively. IC50 against neuraminidase activity for influenza virus infection in host cell 0.82 μM |
Both IgG1 and IgG2b levels were significantly augmented for Th1 humoral immunity modulation by the oral treatment of Mangiferin at 100 mg/kg for 28 days. | Safe and does not display any toxic effect in experimental mice at therapeutic dose. | Not yet recruited in viral diseases or associated disorders. |
Guha et al., 1996; Wang et al., 2011; Li et al., 2007; García et al., 2003 |
Punicalagin (44584733)
|
IC50 against EV71 infection of RD cells was 15 μg/ml and CC50 300 μg/ml. IC50 against DENV-2, RSV and MV was: 7.86±0.40; 0.54±0.04; and 25.49±2.94 (μM) along with CC50 of: 151.44 ± 9.31; 264.83 ± 23.72; 283.76 ± 11.54 μM, Respectively. |
A dosage of 5 mg/kg protected the EV71-infected mice from mortality by 38% without any clinical symptoms after 2 weeks. |
It did not show any toxicity and adverse drug reaction in therapeutic dose (180 mg/kg/day for 90 days) both in clinical and preclinical animal experiment. | In Phase 3 study Pomegranate products were tested for 16-week administering 2 oz. package daily for prevention of Common Cold, Flu or Influenza-Like Symptoms: A Double- Blind, Placebo-Controlled Randomized Clinical Trial, showed impressive results |
Yang et al., 2012; Lin et al., 2013; Patel et al., 2008; https://clinicaltrials.gov/ct2/show/NCT00617318 |
|
Wogonin (5281703) 
|
Nearly 73% inhibition of influenza A virus replication at 10 μg/ml with CC50 using sheep erythrocyte. Docking study suggested strong binding with Mpro catalytic side of SARS-COV-2. |
Dosage of 40 mg/kg significantly reduced liver fibrosis in mice, induced by CCL4. Effect was equivalent to standard. | High therapeutic index and other data suggest that the compound is quite safe and nontoxic in high therapeutic doses and protect the all organ and tissue damage at therapeutic dose | Not yet recruited in viral diseases or associated disorders |
Zhi et al., 2019; Huang et al.,2020; Du et al., 2019 |
Neferine (159654)
|
The EC50 against HIV replication < 0.8 µg/ml with S.I. >8.6. | The increase of MPO activity and fibrosis in lung tissue mediated by Bleomycin were significantly attenuated by n Neferine (20 mg/kg, b.i.d) on 7th and 14th days by reducing inflammatory cytokines and NF-kβ expression. |
Safe, nontoxic to liver and kidney at therapeutic dose | Not yet recruited in viral diseases or associated disorders |
Kashiwada et al., 2005; Zhao et al., 2010 |
Carnosine (439224)
|
IC50 against Dengue and Zika viral protein: 52.3 & 59.5 μM | Inhibited MPO activity, decreased the mortality rate, suppressed TNF-α and IL-1β release, decreased H9N2 viral titer, and in the lungs of infected mice at 10 mg/kg oral dose for 7 days. | Showed minimal cytotoxicity in Huh7 cultured cells. Exogenous carnosine administration up to 2000 mg/kg was well‐tolerated in vivo. |
Not recruited in viral infection patient randomly. | Rothan et al., 2019; Xu et al., 2015 |
Harmine (5280953)
|
EC50 against dual-specific tyrosine phospho-rylation-regulated kinases (DYRKs) of viral replication at early-late stage of HCMV gene expression and HSV at 0.71 μM. Inhibit HSV-1 by blocking IE transcription (Bag et al., 2014). |
Attenuated AG129 mice against EV71 infection by single dose of (12.5 μg/ml) daily i.p. for 4 days. Recover HSV-1 and HSV-2 infected mice (Bag et al., 2014; Bag et al., 2013). |
Very safe on normal mammalian cells at the therapeutic and experimental dose without causing hepatotoxicity, cardiotoxicity and neurotoxicity. | Not yet recruited in viral diseases or associated disorders |
Hutterer et al., 2017; Bag et al., 2013; Bag et al., 2014; Chen et al., 2018 |
