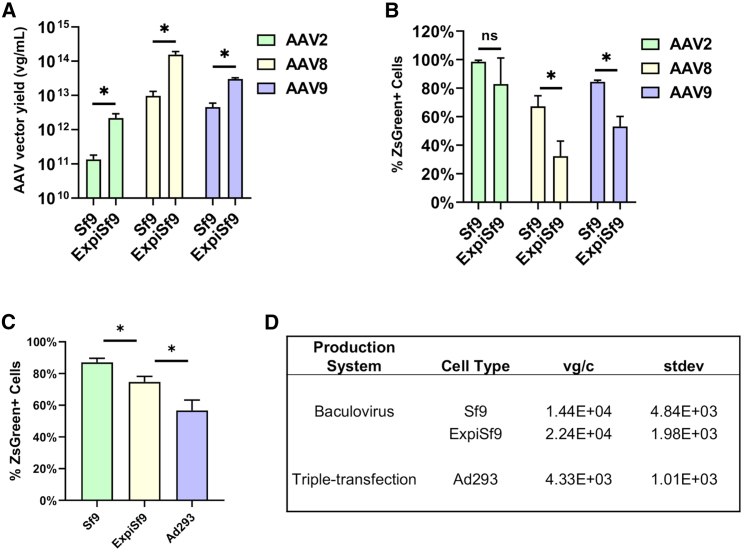
Figure 3.
Viral Genome Titers and Infectivity of Purified AAV Preparations
(A) Purified AAV preparations of various serotypes were produced using either the Bac-to-Bac System in Sf9 cells or the ExpiSf expression system in ExpiSf9 cells, and total viral genomes obtained from 300 mL of culture were measured using qPCR. (B) The infectivity of the purified AAV preparations produced in either Sf9 or ExpiSf9 cells was assessed by measuring the percentage of ZsGreen-positive cells by flow cytometry. ∗p < 0.05. (C) The infectivity of the purified AAV preparations produced in Sf9, ExpiSf9, or Ad293 cells was assessed by measuring the percentage of ZsGreen-positive cells by flow cytometry. ∗p < 0.05. (D) AAV9 yields from the three cell lines tested are shown in terms of viral genomes per cell (vg/c) along with the standard deviation (SD).