Fig. 1. Theoretical model for calculating the number of charged ligands bound to a spherical NP.
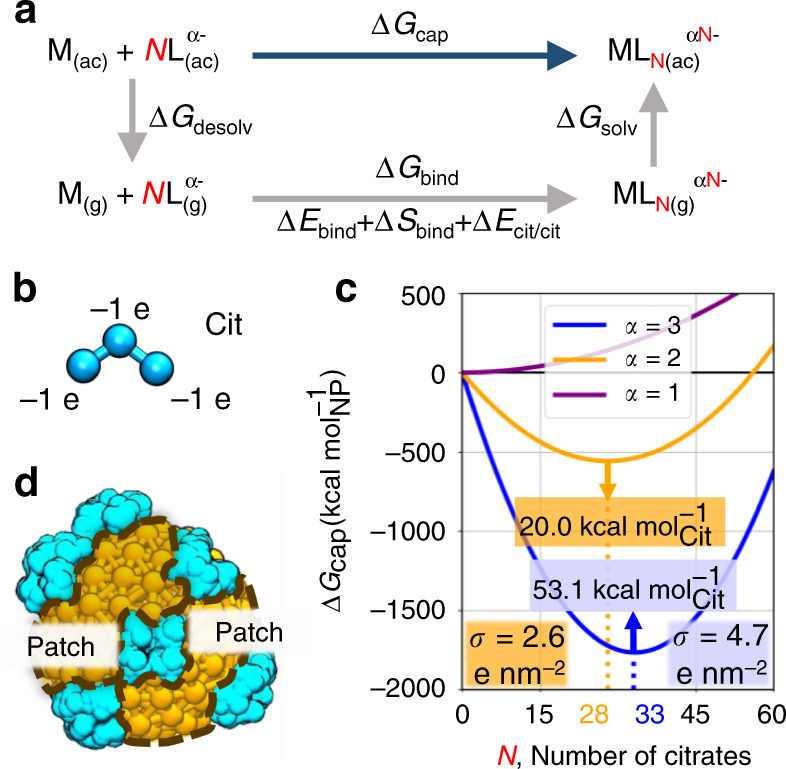
a Thermodynamic cycle on which the proposed theoretical model is based. ΔGcap is obtained in this thermodynamic cycle as ΔGdesolv + ΔGbind + ΔGsolv, where ΔGdesolv is the desolvation energy of the NP core (M) and N ligand molecules (L); ΔGbind is the energy for binding those ligands onto the NP in vacuum; ΔGsolv is the solvation free energy of the resulting capped complex, in which the ligands are modeled as an enveloping uniformly charged sphere. b Explicit coarse-grained model for citrate molecule (Cit), which was parametrized against atomistic MD simulations (see Supplementary Discussion 1f). c Binding energy as a function of N. The model is solved for the three deprotonation states of citrate, i.e., α = 1, 2, 3. The energy minima predict the most likely values for N that, in turn, set the surface charge density (σ) of the NP. d Conceptual illustration of a citrate-capped (cyan) nanoparticle showing the presence of catalytically available hydrophobic patches (brown) as suggested by the estimated surface area coverage.
