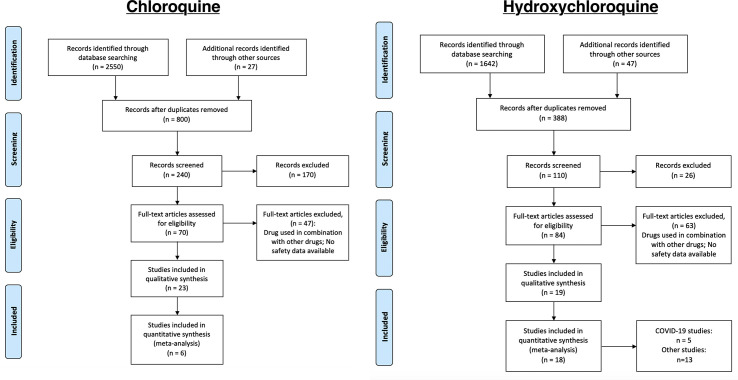
Figure 1.
Process of identifying eligible clinical trials. Records were identified through MEDLINE, CENTRAL, and ClinicalTrials.gov. We used the same process of study collection for both CQ and HCQ. We performed an initial screening, followed by a more stringent screening using our selection criteria. The studies that remained after all the exclusion were the ones used for data extraction. In total, we identified 23 and 17 studies for CQ and HCQ, respectively, which are described in, Table 1 and 2. Of those studies, 6 CQ and 16 HCQ records are controlled RCTs, so we used these studies for our data analysis.