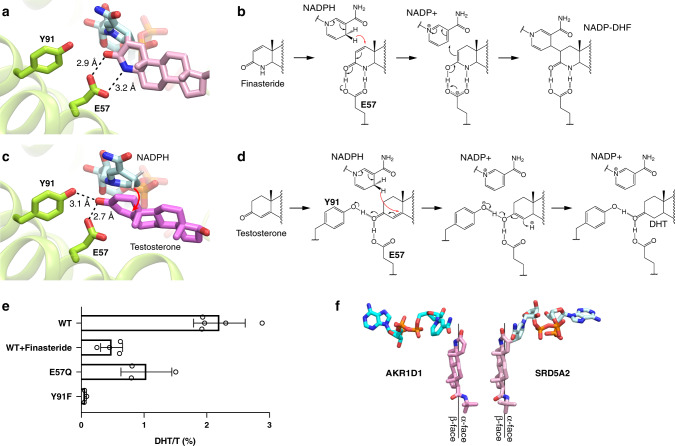
Fig. 3. Mechanisms of SRD5A2 catalysis and inhibition.
a Binding pose of DHF. Y91TM3 is not directly hydrogen bonded to DHF. b Potential mechanism of finasteride inhibition and the covalent adduct formation between NADPH and finasteride. E57TM2 facilitates the hydride transfer to the ∆1,2 bond of finasteride, leading to the formation of a covalent bond. c Binding pose of testosterone based on our docking results. E57TM2 and Y91TM3 each forms a hydrogen bond with the substrate. d Potential mechanism the 5α-reduction of testosterone. E57TM2 and Y91TM3 facilitate hydride transfer to the ∆4,5 bond of testosterone, leading to the formation of DHT. Hydrogen bonds are shown as dashed lines and the hydride transfer is shown as red curved arrows. e Catalysis of testosterone (T) to dihydrotestosterone (DHT) by wild-type SRD5A2 (WT), WT with 500 μM finasteride, and two SRD5A2 mutants E57Q and Y91F. The ratios of DHT to T (DHT/T) were determined by mass spectrometry. All data are presented as the mean ± SEM of three (for E57Q and Y91F), four (for WT + finasteride), and five (for WT) independent experiments. Source data are provided as a Source data file. f Distinct orientations of finasteride relative to NADPH in AKR1D1 and SRD5A2. The finasteride and NADPH conformations in the AKR1D1 structure (PDB ID: 3G1R) are shown by aligning the core ring of finasteride to that of DHF in the SRD5A2 structure.