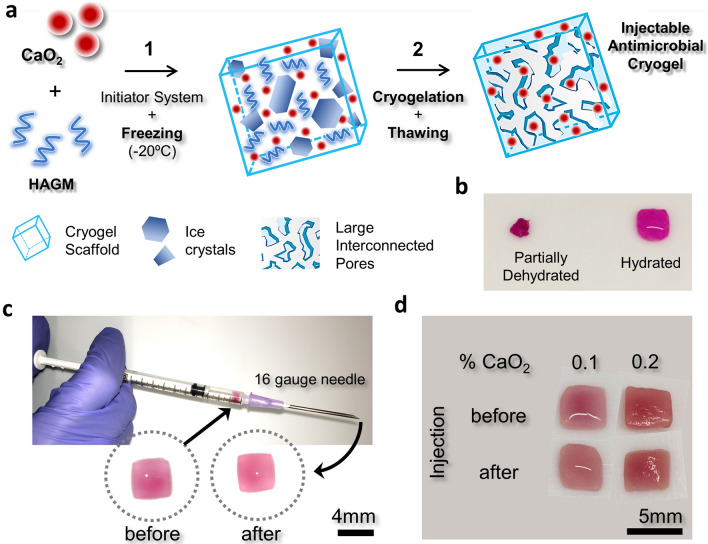
Figure 1.
Engineering antimicrobial and injectable microcomposite cryogels. (a) Overview of the fabrication process of antimicrobial CP-containing injectable cryogels: (1) cryogels were fabricated using 4% HAGM with different amounts of CP (0–0.2% CaO2); an initiator system (APS/TEMED) is added to an aqueous HAGM solution prior to cryopolymerization at − 20 °C. (2) Cryotreatment involves phase separation with ice crystal formation, cross-linking and gelation. Thawing of ice crystals (porogens) results in an interconnected macroporous cryogel network. (b) Cryogel partially dehydrated over Kimwipe regains its original shape and size after hydration. HAGM cryogels were stained with rhodamine for visualization. (c) Following injection through a 16G hypodermic needle, cryogels regain their original shape and dimensions. (d) Cryogels retain their encapsulated CP after needle injection as indicated by the Alizarin Red S staining (n = 5).