Abstract
Background
Gram-negative bacteria mediated gemcitabine resistance in pre-clinical models. We determined if intratumoural lipopolysaccharide (LPS) detection by immunohistochemistry is associated with outcome in advanced pancreatic ductal adenocarcinoma (PDAC) treated with gemcitabine and non-gemcitabine containing 1st-line chemotherapy.
Methods
We examined LPS on tumour tissue from 130 patients treated within the randomised AIO-PK0104 trial and a validation cohort (n = 113) and analysed the association of LPS detection to patient outcome according to treatment subgroups.
Results
In 24% of samples from the AIO-PK0104 study LPS was detected; in LPS-positive patients median OS was 4.4 months, compared to 7.3 months with LPS negative tumours (HR 1.732, p = 0.010). A difference in OS was detected in 1st-line gemcitabine-treated patients (n = 71; HR 2.377, p = 0.002), but not in the non-gemcitabine treatment subgroup (n = 59; HR 1.275, p = 0.478). Within the validation cohort, the LPS positivity rate was 23%, and LPS detection was correlated with impaired OS in the gemcitabine subgroup (n = 94; HR 1.993, p = 0.008) whereas no difference in OS was observed in the non-gemcitabine subgroup (n = 19; HR 2.596, p = 0.219).
Conclusions
The detection of intratumoural LPS as surrogate marker for gram-negative bacterial colonisation may serve as a negative predictor for gemcitabine efficacy in advanced PDAC.
Clinical trial registry
The Clinical trial registry identifier is NCT00440167.
Subject terms: Pancreatic cancer, Predictive markers, Cancer microenvironment, Pancreatic cancer
Background
The majority of patients with pancreatic ductal adenocarcinoma (PDAC) is diagnosed in an advanced stage, resulting in mortality rates almost equal to incidence rates.1 Palliative chemotherapy is considered as an international standard of care for these patients; however, relevant predictive biomarkers for rational treatment decisions are still lacking.2 Increasing evidence indicates a crucial role for the human microbiome in solid malignancies, especially in gastrointestinal tumours which are in close contact to dense microbial colonisation.3,4 Importantly, intratumoural bacteria have been shown to promote tumorigenesis5 and to confer resistance to gemcitabine in human adenocarcinoma.6,7 As reported recently, intratumoural bacteria can metabolise gemcitabine (2′,2′-difluorodeoxycytidine) into its inactive form 2′,2′-difluorodeoxyuridine; in a pre-clinical model, gemcitabine depletion was dependent on the expression of a long isoform of the bacterial enzyme cytidine deaminase (CDDL) commonly found in gram-negative bacteria.7 Bacterial lipopolysaccharide is a major component of the cell wall of gram-negative bacteria and can easily be detected by immunohistochemistry.7
In the present study, we report the results of immunohistochemical detection of intratumoural LPS as a surrogate marker of gram-negative bacterial colonisation in the tumour tissue of 243 patients with advanced PDAC from two independent study cohorts and the correlation of intratumoural LPS detection to patient outcome according to the applied 1st-line chemotherapy regimen (gemcitabine vs non-gemcitabine containing).
Methods
Patients and tumour samples
Archival tumour material was derived from 130 advanced PDAC patients treated within the randomised Phase 3 study AIO-PK0104 (NCT00440167), that compared a 1st-line treatment with gemcitabine + erlotinib versus capecitabine + erlotinib (with the option of a cross-over to the comparator cytotoxic drug in the 2nd-line setting).8 A validation of the LPS results from AIO-PK0104 was performed on 113 patient samples derived from a previously reported prospective biomarker trial,9 in which the 1st-line chemotherapy regimens were as follows: 40 patients received gemcitabine monotherapy, 29 patients gemcitabine and cisplatin, 10 patients gemcitabine and oxaliplatin, 14 gemcitabine and capecitabine, 11 patients single-agent capecitabine, 7 patients capecitabine and oxaliplatin, one patient received FOLFOX-6 and one patient received gemcitabine and 5-FU. Both studies had approval of the local ethics committee and were conducted according to GCP/ICH.
LPS immunohistochemistry
Intratumoural bacterial LPS was detected immunohistochemically on formalin fixed paraffin embedded (FFPE) tumour material as described previously10 using a monoclonal mouse anti-LPS antibody detecting the core bacterial LPS antigen (clone WN1 222-5, Hycult Biotech, Uden, The Netherlands) at a 1:800 dilution. Tumours from 133 patients were included in a tissue microarray (TMA) as three cores of one mm diameter each (71 samples from the AIO-PK0104 cohort, 62 samples from the validation cohort), whereas biopsies from 110 patients were stained as whole-mount tissue sections (59 samples from the AIO-PK0104 cohort, 51 samples from the validation cohort). Normal human colonic mucosa, which physiologically carries high amounts of gram-negative bacteria and normal human liver tissue, which physiologically may show high amounts of LPS, were used as positive controls in each staining run (Supplementary Fig. S1A, B). Of note, normal adjacent pancreatic tissue did not show LPS signals nor unspecific staining in acinar cells (Fig. S1C). On TMA embedded tumour tissue, cases showing strong signals in at least one TMA core or weak and single signals in at least two cores were evaluated as positive, whereas all others were evaluated as negative. On whole-mount tissue sections, the presence of definitive LPS signals throughout the tumour tissue was evaluated as positive, whereas complete absence of signal was evaluated as negative. All slides were read and scored by two researchers (M.G. and S.O.), blinded to the patient outcome and discrepant cases were discussed until agreement was reached. Microphotographs were acquired as described previously.10
Statistical analyses
Progression free survival (PFS) and overall survival (OS) were calculated from the initiation of 1st-line chemotherapy to the occurrence of an event (progress, death). The correlation of tumour or patient characteristics to PFS and OS was calculated using the Kaplan–Meier method. Hazard ratios were estimated by Cox proportional hazards regression. Correlation of patient or sample characteristics was assessed using cross tabulations and two sided χ2-tests. Statistical analyses were run on SPSS software (IBM, Ehningen, Germany).
Results
Patient characteristics
In the overall population (n = 243) median patient age was 62 years; 145 patients were male. On therapy initiation, 23 patients from the AIO-PK0104 trial and 10 patients from the validation cohort had locally advanced disease, whereas all others had distant metastases. Both cohorts were comparable in terms of patient age, sex, KPS groups as well as tissue sample origin and tissue modality (TMA vs whole slide). In the AIO-PK0104 cohort, we detected more locally advanced cases, higher grade tumours and less lung metastases. Related to the trial design, more patients received non-gemcitabine-based 1st-line chemotherapy in the AIO-PK0104 cohort (Table S1). All clinicopathologic patient characteristics and associated survival times of each subgroup are summarised in Table S2.
LPS detection and clinico-pathological patient characteristics
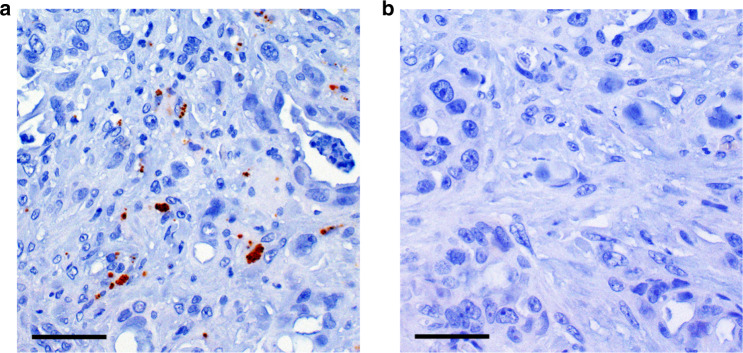
Using immunohistochemistry, bacterial LPS could reliably be detected in 24.5% of all PDAC samples (Fig. 1). To preclude potential sampling errors due to the use of TMA embedded tissue, we compared the staining result in whole-mount tissue sections and the TMA cores of 10 exemplary positive and 15 exemplary negative cases from both cohorts and found complete consistency (Table S3 and Fig. S2A, B). Interestingly, we detected a significantly higher rate of LPS positivity in tumour samples which were derived from metastatic sites compared to primary tumour tissue samples (36.5% vs 9.4%, p < 0.001, Table 1). As expected in a cohort of advanced PDAC patients, tissue samples from liver metastases constituted the largest group (96 of 243 samples) and showed a significantly higher LPS positivity rate than tissue from other metastatic sites or primary tumours (55.2% vs 9.3%, p < 0.001, Table 1). In the subgroup in which only primary tumour samples were examined, no statistically significant association of primary tumour location and LPS detection was found (p = 0.484, Table S4). Moreover, we found no statistically significant association between LPS detection and patient characteristics such as gender, age group or KPS group (Table 1).
Fig. 1. Intratumoural LPS can be detected in advanced PDAC tumour samples.
Immunohistochemical detection of bacterial lipopolysaccharide (LPS) in pancreatic cancer. Exemplary cases of LPS positive (a) and LPS negative (b) pancreatic cancer samples. 200-fold magnification. Scale bars indicate 50 µm.
Table 1.
Association of clinicopathologic variables and LPS detection in the entire study cohort.
| LPS | p-value (χ2-test) | |||
|---|---|---|---|---|
| − | + | Total | ||
| Primary tumour location | ||||
| Unknown | 17 (73.9) | 6 (26.1) | 23 (9.5) | 0.617 |
| Pancreatic head | 103 (74.1) | 36 (25.9) | 139 (57.2) | |
| Pancreatic body | 39 (83.0) | 8 (17.0) | 47 (19.3) | |
| Pancreatic tail | 27 (79.4) | 7 (20.6) | 34 (14.0) | |
| Total | 186 (76.5) | 57 (23.5) | 243 (100.0) | |
| Metastasis at therapy initiation | ||||
| Lung | 5 (83.3) | 1 (16.7) | 6 (2.5) | 0.023 |
| Liver | 65 (67.7) | 31 (32.3) | 96 (39.5) | |
| Peritonuem | 12 (100.0) | 0 (0.0) | 12 (4.9) | |
| Other | 16 (69.6) | 7 (30.4) | 23 (9.5) | |
| None | 32 (97.0) | 1 (3.0) | 33 (13.6) | |
| Liver and peritoneum | 17 (94.4) | 1 (5.6) | 18 (7.4) | |
| Lung and liver | 6 (54.5) | 5 (45.5) | 11 (4.5) | |
| Liver and bone | 1 (100.0) | 0 (0.0) | 1 (0.4) | |
| Lung and abdominal lymph nodes | 3 (100.0) | 0 (0.0) | 3 (1.3) | |
| Liver and lymph nodes | 16 (72.7) | 6 (27.3) | 22 (9.1) | |
| Liver, lung and lymph nodes | 3 (75.0) | 1 (25.0) | 4 (1.6) | |
| Lymph nodes | 8 (72.7) | 3 (27.3) | 11 (4.5) | |
| Liver and adrenal gland | 1 (50.0) | 1 (50.0) | 2 (0.8) | |
| Lung, liver and bone | 1 (100.0) | 0 (0.0) | 1 (0.4) | |
| Total | 186 (76.5) | 57 (23.5) | 243 (100.0) | |
| Tissue origin | ||||
| Hepatic metastasis | 59 (57.3) | 44 (42.7) | 103 (42.4) | 0.000 |
| Primary tumor | 106 (90.6) | 11 (9.4) | 117 (48.1) | |
| Peritoneal metastasis | 8 (100.0) | 0 (0.0) | 8 (3.3) | |
| Lung metastasis | 5 (83.3) | 1 (16.7) | 6 (2.5) | |
| Lymph node | 4 (100.0) | 0 (0.0) | 4 (1.6) | |
| Other/unknown | 4 (80.0) | 1 (20.0) | 5 (2.1) | |
| Total | 186 (76.5) | 57 (23.5) | 243 (100.0) | |
| Age group | ||||
| ≤60 | 75 (73.5) | 27 (26.4) | 102 (42.0) | 0.346 |
| >60 | 111 (77.6) | 30 (22.4) | 141 (58.0) | |
| Total | 186 (76.5) | 57 (23.5) | 243 (100.0) | |
| Gender | ||||
| Male | 112 (77.2) | 33 (22.8) | 145 (59.7) | 0.755 |
| Female | 74 (75.5) | 24 (24.5) | 98 (40.3) | |
| Total | 186 (76.5) | 57 (23.5) | 243 (100.0) | |
| KPS group | ||||
| ≤80 | 75 (72.8) | 28 (27.2) | 103 (42.7) | 0.210 |
| > 80 | 110 (79.7) | 28 (20.3) | 138 (57.3) | |
| Total | 185 (76.8) | 56 (23.2) | 241 (100.0) | |
| CTX type | ||||
| Non-gemcitabine 1st line subgroup | 64 (82.1) | 14 (17.9) | 78 (32.1) | 0.164 |
| Gemcitabine 1st line subgroup | 122 (73.9) | 43 (26.1) | 165 (67.9) | |
| Total | 186 (76.5) | 57 (23.5) | 243 (100.0) | |
| Disease stage at therapy initiation | ||||
| Locally advanced | 32 (97.0) | 1 (3.0) | 33 (13.6) | 0.003 |
| Metastatic | 154 (73.3) | 56 (26.7) | 210 (86.4) | |
| Total | 186 (76.5) | 57 (23.5) | 243 (100.0) | |
| Grade group | ||||
| G1-G2 | 75 (84.3) | 14 (15.7) | 89 (36.6) | 0.031 |
| G3-G4 | 111 (72.1) | 43 (27.9) | 154 (63.4) | |
| Total | 186 (76.5) | 57 (23.5) | 243 (100.0) | |
Intratumoural LPS detection is associated with inferior survival in gemcitabine-treated PDAC patients
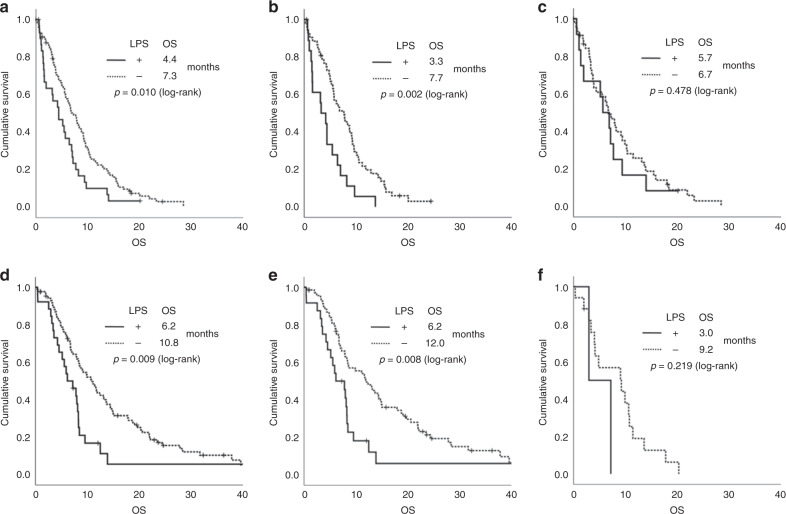
We detected bacterial LPS in the tumour material of 24% of the 130 patients from the AIO-PK0104 study, which was significantly associated to inferior OS (4.4 vs 7.3 months, HR 1.732, p = 0.010, Fig. 2a). Intratumoural LPS was significantly associated with a shorter OS in the 1st-line gemcitabine randomisation arm (3.3 vs 7.7 months, HR 2.377, p = 0.002, Fig. 2b), whereas no such difference in the 1st-line capecitabine arm was detected (5.7 vs 6.7 months, HR 1.275, p = 0.478, Fig. 2c). All 113 patients selected for the validation cohort received erlotinib-free, either gemcitabine-based or non-gemcitabine-based 1st-line chemotherapy regimens9; 23% of these tumour samples were LPS positive, which again was significantly associated to shorter patient PFS (4.1 vs 7.8 months, HR 1.760, p = 0.028) and OS (6.2 vs 10.8 months, HR 1.880, p = 0.009, Fig. 2d). In subgroup analyses, the negative impact of LPS detection on either PFS (HR 2.051, p = 0.010) or OS (HR 1.993, p = 0.008, Fig. 2e) was restricted to the gemcitabine subgroup, whereas no difference in PFS (HR 1.292, p = 0.742) or OS (HR 2.596, p = 0.219, Fig. 2f) was detected for the non-gemcitabine subgroup with regard to LPS status, respectively (Table 2).
Fig. 2. Intratumoural LPS is associated with inferior OS in gemcitabine-treated PDAC patients.
Overall survival (OS) of each patient subgroup according to intratumoural LPS detection. Kaplan–Meier plots of OS in the a AIO-PK0104 overall cohort. b 1st-line gemcitabine subgroup of the AIO-PK0104 trial population. c 1st-line capecitabine subgroup of the AIO-PK0104 trial population. d Validation overall cohort. e 1st-line gemcitabine subgroup of the validation cohort. f 1st-line non-gemcitabine subgroup of the validation cohort. Crossed lines indicate censored cases.
Table 2.
Patient prognosis according to LPS detection in each patient subgroup.
| LPS | n | % | OS (months) | p (log-rank) | HR | 95% CI | PFS (months) | p (log-rank) | HR | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients | |||||||||||
| Total | + | 57 | 24.5 | 5.6 | 0.000 | 1805 | 1.312–2.482 | 3.5 | 0.034 | 1413 | 1.024–1.951 |
| − | 186 | 76.5 | 8.5 | 4.0 | |||||||
| AIO-PK0104 | + | 31 | 23.9 | 4.4 | 0.010 | 1732 | 1.133–2.649 | 1.8 | 0.249 | 1278 | 0.840–1.944 |
| − | 99 | 76.2 | 7.3 | 2.8 | |||||||
| Validation cohort | + | 26 | 23.0 | 6.2 | 0.009 | 1880 | 1.161–3.045 | 4.1 | 0.028 | 1760 | 1.055–2.936 |
| − | 87 | 77.0 | 10.8 | 7.8 | |||||||
| 1st line gemcitabine subgroup | |||||||||||
| Total | + | 43 | 35.2 | 5.5 | 0.000 | 2.081 | 1.425–3.038 | 3.7 | 0.005 | 1.728 | 1.175–2.542 |
| − | 122 | 64.8 | 8.8 | 6.1 | |||||||
| AIO-PK0104 | + | 19 | 26.8 | 3.3 | 0.002 | 2.377 | 1.353–4.178 | 3.2 | 0.062 | 1.681 | 0.968–2.919 |
| − | 52 | 73.2 | 7.7 | 3.6 | |||||||
| Validation cohort | + | 24 | 25.5 | 6.2 | 0.008 | 1.993 | 1.190– 3.338 | 4.7 | 0.010 | 2.051 | 1.177–3.573 |
| − | 70 | 74.5 | 12.0 | 9.2 | |||||||
| 1st line non-gemcitabine subgroup | |||||||||||
| Total | + | 14 | 18.0 | 5.7 | 0.306 | 1.373 | 0.747– 2.526 | 2.2 | 0.955 | 1.018 | 0.554–1.868 |
| − | 64 | 82.0 | 7.3 | 2.2 | |||||||
| AIO-PK0104 | + | 12 | 20.3 | 5.7 | 0.478 | 1.275 | 0.651– 2.497 | 1.7 | 0.829 | 0.929 | 0.475–1.817 |
| − | 47 | 79.7 | 6.7 | 2.2 | |||||||
| Validation cohort | + | 2 | 10.5 | 3.0 | 0.219 | 2.596 | 0.536– 12.580 | 2.2 | 0.742 | 1.292 | 0.278–6.014 |
| − | 17 | 89.5 | 9.2 | 2.7 | |||||||
Median times of overall survival (OS) and progression free survival (PFS) according to intratumoural LPS detection in each study subgroup as well as corresponding hazard ratios (HR) and 95% confidential intervals (95% CI).
In Cox multivariate regression analyses adjusting for the parameters which were statistically significant prognosticators in univariate analyses (tumour grade, KPS and disease stage at chemotherapy initiation), we confirmed LPS detection as independent negative predictor for both PFS and OS in the gemcitabine 1st-line chemotherapy subgroup (Table 3).
Table 3.
Intratumoural LPS is an independent negative prognostic biomarker in gemcitabine-treated PDAC patients.
| OS | ||||
|---|---|---|---|---|
| parameter | p (Cox) | HR | 95% CI | |
| All cases | Grade group | 0.002 | 1.592 | 1.194–2.122 |
| KPS group | 0.000 | 0.601 | 0.455–0.793 | |
| LPS | 0.002 | 1.664 | 1.203–2.302 | |
| Gemcitabine 1st line subgroup | Grade group | 0.000 | 1.977 | 1.401–2.790 |
| Disease stage | 0.029 | 1.877 | 1.065–3.306 | |
| LPS | 0.002 | 1.872 | 1.267–2.765 | |
| Non-gemcitabine 1st line subgroup | KPS group | 0.000 | 0.401 | 0.246–0.654 |
| PFS | ||||
|---|---|---|---|---|
| Parameter | p (Cox) | HR | 95% CI | |
| All cases | Grade group | 0.002 | 1.610 | 1.187–2.186 |
| KPS group | 0.002 | 0.637 | 0.476–0.853 | |
| CTX type | 0.000 | 0.456 | 0.335–0.620 | |
| Gemcitabine 1st line subgroup | LPS | 0.009 | 1.674 | 1.136–2.467 |
| Grade group | 0.001 | 1.838 | 1.275–2.648 | |
| Non-gemcitabine 1st line subgroup | KPS group | 0.002 | 0.435 | 0.258–0.733 |
Cox regression analyses in the indicated patient subgroups for overall survival (OS) and (PFS), adjusting for tumour grade, KPS group, type of 1st-line palliative chemotherapy and disease stage at 1st-line therapy initiation.
KPS: Karnofsky performance status; tumour grade: high grade (G3-G4) vs low grade (G1-G2) differentiation; CTX-type: type of 1st-line chemotherapy (gemcitabine-based vs non-gemcitabine based); disease stage: disease stage at 1st-line therapy initiation (metastatic vs locally advanced).
To preclude a potential impact of the imbalance of LPS positivity in metastatic and primary tumour tissue, we compared OS and PFS according to LPS detection in each subgroup and obtained similar results as in the overall cohorts (Table S5). Similarly, we tested whether tissue modality (whole-mount sections vs. TMA) affected the association of LPS detection and patient outcome. Although we detected significantly different positivity rates in both subgroups, LPS positivity remained a negative predictor for outcome in gemcitabine-based 1st-line treated patients, whereas we detected no significant differences in non-gemcitabine-treated patients (Table S6).
Intratumoural LPS detection is associated with inferior tumour therapy response in gemcitabine-treated PDAC patients
From 110 patients treated within the AIO-PK0104 study, objective tumour response data (a secondary trial endpoint) was available, but we did not detect a significant correlation of LPS positivity and tumour response estimated radiologically by RECIST (Table S7). As the reliability of radiologic assessment of tumour response in PDAC remains a matter of debate,11 we additionally assessed the biochemical treatment response by CA19-9 serum levels. Data on baseline CA19-9 levels and after the first or third chemotherapy cycle (eight weeks for the gemcitabine 1st-line subgroup, nine weeks for the capecitabine 1st-line subgroup8) were available from 95 patients in the AIO-PK0104 cohort. By defining a biochemical response as drop in CA19-9 levels of at least 20% from baseline levels,12 only 2 patients with LPS-positive tumours in the gemcitabine 1st-line subgroup (n = 54) showed a CA 19-9 response, whereas 10 patients with LPS-positive tumours were non-responders. Within the non-gemcitabine 1st-line subgroup (n = 41) no significant association between LPS status and CA 19-9 response was detected (for details see Table 4).
Table 4.
LPS positivity is associated with a lower biochemical response in gemcitabine-treated patients.
| LPS | ||||
|---|---|---|---|---|
| − | + | Total | p (χ2) | |
| Gemcitabine 1st line subgroup | ||||
| CA 19-9 non-responder | 20 | 10 | 30 | 0.028 |
| CA 19-9 responder | 22 | 2 | 24 | |
| Total | 42 | 12 | 54 | |
| Non-gemcitabine 1st line subgroup | ||||
| CA 19-9 non-responder | 20 | 4 | 24 | 0.585 |
| CA19-9 responder | 13 | 4 | 17 | |
| Total | 33 | 8 | 41 | |
Cross-tabs calculation of biochemical treatment response according to LPS status in 95 patients from the AIO-PK0104 cohort, defining a drop in CA19-9 of at least 20% compared to baseline level after the first chemotherapy cycle as ‘CA 19-9 response’.
Discussion
The impact of the tumour microbiome on patient outcome and therapy response in several human solid malignancies has recently gained increasing attention.3,13 In far contrast to other malignancies like colorectal or lung cancer, biomarkers that predict treatment efficacy of specific drugs are still lacking in PDAC. In 2017, Geller et al. reported pre-clinical data suggesting that intratumoural bacteria expressing CDDL mediate tumour resistance to gemcitabine.7 However, as to our best knowledge, these data have never been clinically validated yet. As gemcitabine is mainly applied in PDAC, we thought to validate the hypotheses from Geller and co-workers on patient samples from our previously conducted clinical trials in advanced PDAC.8,9 As both the AIO-PK0104 cohort as well as the validation cohort contained patients treated with 1st-line gemcitabine and also non-gemcitabine containing chemotherapy, we thought to evaluate a potential predictive role for the occurrence of intratumoural bacteria on gemcitabine efficacy.
Unfortunately, no positivity rates of LPS immunohistochemistry were reported in the study by Geller et al., but the rate of 25% LPS positivity in our overall study population was significantly lower than the rate of 76% ‘bacterial DNA’-positive PDAC samples reported in their study.7 However, as their molecular assay detects all kinds of bacteria with superior sensitivity compared to immunohistochemistry and LPS is a cell wall component of gram-negative bacteria only, both figures are not directly comparable. One also might expect higher positivity rates examining whole-mount primary tumour resection specimens—as performed by Geller et al.—compared to biopsy tissue or TMA embedded tumour material. Nevertheless, we precluded a potential bias resulting from tissue modality and confirmed the reliability of TMA embedded tissue to detect intratumoural LPS compared to whole-mount tissue. Moreover, although our study population consisted of patients with advanced PDAC, where histological confirmation is often performed by a percutaneous biopsy of metastases,9 we even detected higher positivity rates in metastases than in primary tumours, which was mostly due to more LPS-positive liver metastases. However, intratumoural LPS detection remained a potential negative predictor of gemcitabine efficacy irrespective of the tissue origin subgroup or the modality of the tissue examined (whole-mount sections vs TMA).
Our main results (summarised within Table 2) provide evidence that LPS detection in FFPE tumour samples may serve as a negative predictor for gemcitabine efficacy in advanced PDAC, thereby confirming for the first time the previously published pre-clinical data.7 This potential predictive biomarker for gemcitabine efficacy may be of special interest, as additive antibiotic treatment in LPS-positive cases could at least theoretically be used to eliminate bacterial colonisation and thus overcome bacterially mediated chemotherapy resistance as already shown in vivo.7 Although gemcitabine monotherapy has been largely replaced by more efficient chemotherapy regimens in the clinically fit patient,14 it is still widely used in the clinical practice, especially in patients with significant co-morbidities,15 where adjuvant antibiotic treatment in LPS-positive tumours could increase the unfortunately still suboptimal treatment efficacy.
Our study has several limitations that have to be taken into account when interpreting the results: the design was retrospective and a relevant heterogeneity on the applied treatment regimens (specifically in the validation cohort) may lead to a potential bias. In addition, in both cohorts the use of 2nd-line therapy (usually with the alternative cytotoxic backbone, e.g. 5-fluorouracil-based after gemcitabine and vice versa) may have an impact on the results, specifically for OS. However, as the rate of 2nd-line therapy was below 50% in AIO-PK0104, a significant impact of 2nd- and further-line treatments on our results should not be expected. It must also be noted that all patients included in this LPS analysis were treated before the introduction of FOLFIRINOX16 or gemcitabine/nab-paclitaxel.17 In addition, the LPS immunohistochemistry used only detects a cell wall component of gram-negative bacteria and does not allow assessment of bacterial viability or further characterisation of the bacterial infiltrate. Future studies using more specific methods such as 16s-RNA-FISH or 16s-RNA gene sequencing could complement our rather broad-based approach and specify if it is indeed CDDL expressing bacteria which are associated to worse outcome in gemcitabine- treated patients with LPS-positive tumours. Moreover, although our findings indicate a pivotal role of gram-negative bacteria in mediating gemcitabine resistance, we cannot preclude that we detected a mere surrogate marker, as the impact of the tumour microbiome on chemotherapy resistance may be multifactorial and its effect may not be limited to gemcitabine. For instance, the gram-negative bacteria Fusobacterium nucleatum has been shown to induce resistance to platinum-based chemotherapy in colorectal cancer via autophagy induction.18 However, as Fusobacterium nucleatum has a relatively low abundance in PDAC compared to other gram-negative bacteria,7,13 a significant impact on the few patients which received platinum compounds in our study is not to be expected.
As both AIO-PK0104 as well as the prospective biomarker study (used for the validation cohort) unfortunately had not collected information about the frequency of endoscopic retrograde cholangiopancreatography (ERCP) including placement of bile duct stents, we are not able to provide data on a potential correlation of the ERCP procedure (and/or the occurrence of cholangitis and subsequent use of antibiotics) in our patient population—aspects that of course would be of additional scientific interest in interpreting the reported data. Future studies on the role of the microbiome in pancreatic cancer should not only focus on potential sources, such as biliary or pancreatic duct instrumentations and the local microbiome in other locations throughout the body, but also on the effect of antibiotic treatments. Thus, examining the microbiome in liquid or solid biopsies may eventually be used to guide informed tumour therapy.
Nevertheless, based on the results from AIO-PK0104 and the validation cohort, intratumoural bacterial LPS detection in PDAC samples may serve as a novel negative predictive biomarker for gemcitabine efficacy, confirming previous pre-clinical observations. These findings must be validated prospectively and should be addressed in the light that this biomarker also may be modifiable by the use of antibiotics.
Supplementary information
Acknowledgements
We would like to thank all patients and their families, nurses, study coordinators and AIO investigators for their active participation in the clinical trials that contributed data to this translational biomarker study. We also thank A. Sendelhofert and A. Heier for excellent technical assistance. An earlier preliminary version of this paper was deposited on the preprint server medRxiv: Guenther, M., et al. Bacterial lipopolysaccharide as negative predictor of gemcitabine efficacy in advanced pancreatic cancer * translational results from the AIO-PK0104 phase 3 study. medRxiv. 2020:2020.05.01.20087668. https://www.medrxiv.org/content/10.1101/2020.05.01.20087668v1.
Author contributions
M.G. and S.O. read and scored immunohistochemical stainings, performed statistical analyses and drafted the figures. S.O. and S.B. drafted the paper. T.K. read slides and provided material. M.H., S.K., C.B.W., V.H., M.v.B-B., J.M., J.W. and S.B. provided clinical patient data and patient material. All authors read and reviewed the paper.
Ethics approval and consent to participate
In both study cohorts, the local ethics committee of the faculty of medicine of Ludwig-Maximilians-University Munich approved the use of patient material for translational research purposes (project numbers 554-11 and 401-15). All patients gave written informed consent to participate in their respective study upon study enrolment. This study was performed in accordance with the Declaration of Helsinki.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information file.
Competing interests
The authors declare no competing interests in relation to the work described within the present paper.
Funding information
This study was financed by internal funds of the Institute of Pathology, LMU.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Michael Guenther, Michael Haas, Stefan Boeck, Steffen Ormanns
Change history
6/10/2021
A Correction to this paper has been published: 10.1038/s41416-021-01447-1
Supplementary information
Supplementary information is available for this paper at 10.1038/s41416-020-01029-7.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: A Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018;15:333. doi: 10.1038/s41575-018-0005-x. [DOI] [PubMed] [Google Scholar]
- 3.Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178:795–806. e12. doi: 10.1016/j.cell.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helmink B. A., Khan M. W., Hermann A., Gopalakrishnan V., Wargo J. A. The microbiome, cancer, and cancer therapy. Nat. Med. 25, 377–388 (2019) [DOI] [PubMed]
- 5.Sethi V, Kurtom S, Tarique M, Lavania S, Malchiodi Z, Hellmund L, et al. Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology. 2018;155:33–37. e6. doi: 10.1053/j.gastro.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehouritis P, Cummins J, Stanton M, Murphy CT, McCarthy FO, Reid G, et al. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci. Rep. 2015;5:14554. doi: 10.1038/srep14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–160.. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinemann V, Vehling-Kaiser U, Waldschmidt D, Kettner E, Märten A, Winkelmann C, et al. Gemcitabine plus erlotinib followed by capecitabine versus capecitabine plus erlotinib followed by gemcitabine in advanced pancreatic cancer: final results of a randomised phase 3 trial of the ‘Arbeitsgemeinschaft Internistische Onkologie’(AIO-PK0104) Gut. 2013;62:751–759. doi: 10.1136/gutjnl-2012-302759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas M, Ormanns S, Baechmann S, Remold A, Kruger S, Westphalen CB, et al. Extended RAS analysis and correlation with overall survival in advanced pancreatic cancer. Br. J. Cancer. 2017;116:1462. doi: 10.1038/bjc.2017.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ormanns S, Altendorf-Hofmann A, Jackstadt R, Horst D, Assmann G, Zhao Y, et al. Desmogleins as prognostic biomarkers in resected pancreatic ductal adenocarcinoma. Br. J. Cancer. 2015;113:1460. doi: 10.1038/bjc.2015.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baliyan V, Kordbacheh H, Parakh A, Kambadakone A. Response assessment in pancreatic ductal adenocarcinoma: role of imaging. Abdom. Radiol. 2018;43:435–444.. doi: 10.1007/s00261-017-1434-7. [DOI] [PubMed] [Google Scholar]
- 12.Boeck S, Stieber P, Holdenrieder S, Wilkowski R, Heinemann V. Prognostic and therapeutic significance of carbohydrate antigen 19-9 as tumor marker in patients with pancreatic cancer. Oncology. 2006;70:255–264. doi: 10.1159/000094888. [DOI] [PubMed] [Google Scholar]
- 13.Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, et al. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science. 2020;368:973–80.. doi: 10.1126/science.aay9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Springfeld C, Jäger D, Büchler MW, Strobel O, Hackert T, Palmer DH, et al. Chemotherapy for pancreatic cancer. La Presse Médicale. 2019;48:e159–e74.. doi: 10.1016/j.lpm.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Macarulla T, Carrato A, Díaz R, García A, Laquente B, Sastre J, et al. Management and supportive treatment of frail patients with metastatic pancreatic cancer. J. Geriatr. Oncol. 2019;10:398–404. doi: 10.1016/j.jgo.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 17.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170:548–563. e16. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information file.