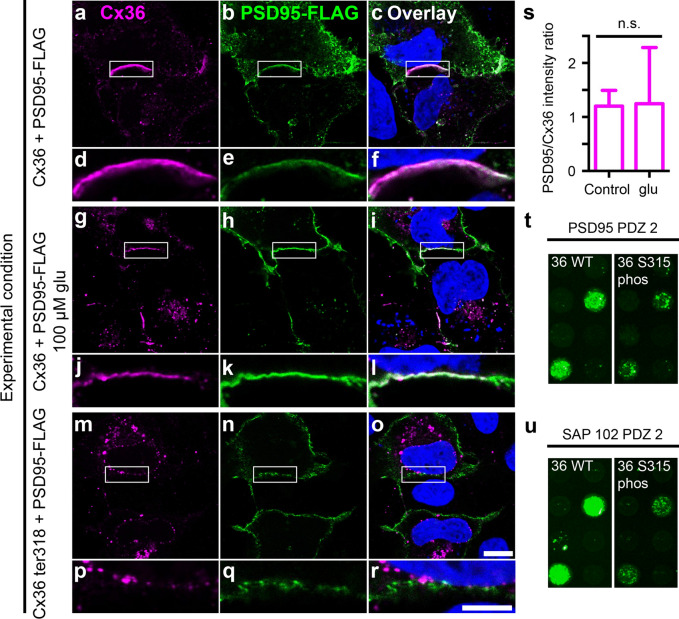
Figure 5.
Activation of CaMKII does not cause any apparent changes in Cx36/PSD95 association. (a–f) Cx36 and PSD-95-FLAG colocalized at gap junctions in transfected HeLa cells. (g–l) Activation of CaMKII by treatment with glutamate (glu) did not affect the localization of PSD95 at gap junctions. (m–r) Expression of the Cx36 S318 ter mutant prevented PSD95 binding, indicating that this interaction requires a PDZ domain. Please note that hardly any gap junctions were formed and colocalization was absent also from intracellular vesicles, which showed colocalization when the PDZ domain of mmCx36 was still present (a–l). (s) Activation of CaMKII via glutamate (glu) did not affect the PSD95/Cx36 intensity ratio at gap junctions. 12 gap junctions in each condition were quantified. (t, u) Microarrays revealed phosphorylation-dependent reduction of mmCx36 C-terminal binding to PSD95 PDZ2 and SAP102 PDZ2. Scale: 10 µm.