Abstract
Splenosis is defined as the autotransplantation of viable splenic tissue throughout various anatomic compartments. Intrahepatic splenosis (IHS) is rare and diagnosis is often challenging. This study aims to provide a comprehensive review on IHS. A literature review was performed on PubMed database. Fifty-six articles with 59 reported cases were included. The majority of the patients were male (n = 49, 83.1%). Median age was 51 years. Risk factors for hepatocellular carcinoma (HCC) included hepatitis B (n = 8, 13.6%) and cirrhosis (n = 12, 20.3%). The majority of the patients were asymptomatic (62.7%) and did not have risk factors for HCC (55.9%). We report a diagnostic triad for IHS: 1) previous history of abdominal trauma or splenectomy, 2) absence of risk factors for liver malignancy and 3) typical imaging features. Non-invasive diagnostic tests such as technetium-99m-tagged heat-damaged red blood cell scintigraphy are useful in diagnosis. Malignancy should be ruled out in the presence of risk factors for HCC.
Keywords: intrahepatic splenosis, hepatocellular carcinoma, splenectomy, liver tumour, liver mass
Introduction
Splenosis was first described by Albrecht in 1896 and subsequently named by Buchbinder and Lipkoff in 1939 [1]. Splenosis is defined as the autotransplantation of viable splenic tissue throughout various anatomic compartments of the body. Previous splenectomy, abdominal trauma or splenic rupture predisposes to splenosis [2]. Intra-abdominal splenosis involving the serosal surface of the small or large bowel, parietal peritoneum and mesentery is relatively common [3]. However, intrahepatic splenosis (IHS) is rare, with many authors quoting fewer than 50 cases published to date [4-6]. Diagnosis of IHS is often challenging as patients are often asymptomatic or present with non-specific abdominal pain, and radiological imaging findings may resemble other hepatic lesions, particularly hepatocellular carcinoma (HCC), adenoma and focal nodular hyperplasia (FNH). With the increase in abdominal imaging for patients with vague abdominal symptoms and better quality of imaging technology, incidental liver lesions are common. Once a liver lesion is detected, a clinician is faced with a challenge to diagnose the lesion with certainty with the primary goal of ruling out a malignancy. IHS is a benign condition and does not warrant surveillance or intervention unless the patient is severely symptomatic. Definitive diagnosis of IHS is possible with percutaneous needle biopsy, intra-operative frozen section or post-operative histopathological analysis or technetium-99m-tagged (Tc-99m) heat-damaged red blood cell (RBC) scintigraphy. However, patients undergoing additional diagnostic tests may bear unnecessary costs and morbidity. This is compounded by anxiety associated with the waiting interval or knowledge of false negative reports. Hence it is important to understand this pathological condition and its clinical features. To date, there are two literature reviews on IHS which summarize reported cases [4, 7]. However, these reviews do not include the clinical presentation, presence of risk factors for malignancy, laboratory investigations and imaging characteristics. This study aims to provide a comprehensive overview on IHS.
Material and methods
A literature review was performed on PubMed database for the keywords “intrahepatic splenosis” OR “hepatic splenosis” from the period of 1939 to 2019. The last search was performed on 18 January 2020. The search yielded 81 articles: 11 articles were not in English, 6 articles were not case reports or series, 5 articles included isolated extrahepatic splenosis, 1 article was on splenosis in animals, 1 article included an incidental finding of splenosis on autopsy, and the full text was not available for 1 article. The remaining 56 articles were included in the analyses, with a total of 59 reported cases (Table 1) [4-59]. Year of study, age, sex, reason for splenectomy, time from splenectomy to presentation, presence of risk factors for HCC, clinical presentation, laboratory investigation results, imaging features, initial differential diagnoses and method of confirming diagnosis were extracted from the articles. Figure 1 is a graphical representation of the trend of reporting of cases of IHS, which shows an increasing trend in reporting.
Table 1.
Summary of 59 reported cases of intrahepatic splenosis from 1939 to 2019
| No. | Year | First author | Age/ Sex | Reason for splenectomy | Time* (years) | Risk factor for HCC | Clinical presentation | Laboratory investigations# | No. of lesions | Location | Size (cm) | Initial diagnosis | Confirmatory diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1993 | Yoshimitsu [8] | 51/F | Banti syndrome | 23 | Cirrhosis | Asymptomatic | ALP elevated | 1 | S3 | 2.5 | HCC | Surgery (liver resection) |
| 2 | 1997 | Gruen [9] | 38/F | Trauma | 20 | Fatty liver | Asymptomatic | ALT, AST, ALP, bilirubin elevated | 1 | S3, S4 | 3.9 | HCC/FNH | Surgery (liver resection) |
| 3 | 1998 | D’Angelica [10] | 38/F | Trauma | 20 | Alcohol | Asymptomatic | ALT, AST, ALP, GGT, bilirubin elevated | 1 | S3, S4 | 2.5 | Adenoma/FNH | Surgery (liver resection) |
| 4 | 1999 | Foroudi [11] | 59/F | NM | 47 | Nil | Upper abdominal pain and back pain | Normal | Multiple | Right lobe | NM | Liver metastasis | Tc-99m DRBC |
| 5 | 2000 | De Vuysere [12] | 50/M | Trauma | 34 | Nil | Epigastric pain | Normal | Multiple | S2 | 6 | Hepatic splenosis | Surgery (biopsy) |
| 6 | 2002 | Gamulin [13] | 49/M | Trauma | 37 | Nil | Asymptomatic | Normal | 1 | Left lobe | 6.6 × 4.2 | B-cell lymphoma | Surgery (explorative laparotomy) |
| 7 | 2002 | Lee [14] | 43/M | Trauma | 20 | HBV Cirrhosis | Asymptomatic | Normal, except for INR | 1 | S6 | 3.5 | HCC | Surgery (liver resection) |
| 8 | 2002 | Pekkafali [15] | 21/M | Trauma | 15 | Nil | Epigastric pain | Normal | 1 | Left lobe | 3.4 × 2.3 | Hepatic splenosis | Tc-99m DRBC |
| 9 | 2003 | Kim [16] | 43/M | Trauma | 21 | HBV Cirrhosis | Asymptomatic | Normal | 1 | S6 | 3 | HCC | Surgery (liver resection) |
| 10 | 2004 | Di Costanzo [17] | 58/M | Trauma | 46 | HBV Cirrhosis | Abdominal pain | AFP elevated | 1 | S2 | 4.8 | HCC | Needle biopsy, Tc-99m DRBC |
| 11 | 48/F | Trauma | 41 | HCV Cirrhosis | Asymptomatic | ALT, AST and AFP elevated | 1 | S3 | 3.1 | HCC | US-guided biopsy | ||
| 12 | 2004 | Kondo [18] | 55/M | Trauma | 31 | HCV | Asymptomatic | NM | 1 | S7 | 3.5 | HCC/FNH/ haemangioma | US-guided percutaneous biopsy |
| 13 | 2006 | Ferraioli [19] | 40/M | Trauma | 28 | HCV | Asymptomatic | Normal | 1 | S7 | 6 × 3.1 | Hepatic splenosis | US-guided biopsy |
| 14 | 2008 | Choi [20] | 32/M | Trauma | 26 | HBV carrier | Asymptomatic | AST elevated | Multiple | S4a, S6 | 1.0-3.0 | HCC | Surgery (explorative laparotomy) |
| 15 | 2008 | Grande [21] | 41/M | Trauma | 35 | Nil | Asymptomatic | Normal | Multiple | S7 | 0.5-4.5 | Hepatic splenosis | Tc-99m DRBC |
| 16 | 2008 | Imbriaco [22] | 39/M | Trauma | 24 | Nil | Abdominal pain | NM | Multiple | Left and right lobes, pancreatic tail, adjacent to upper pole of left | 3.0 | Neoplasm | Surgery (explorative laparotomy) |
| 17 | 2008 | Lu [23] | 59/M | Trauma | NM | HBV | Asymptomatic | Normal | Multiple | S7, left lobe | 1.2-2.2 | Hepatic splenosis | Tc-99m DRBC |
| 18 | 2008 | Nakajima [24] | 41/M | Trauma | 21 | Nil | Incidental finding on work-up for acute enteritis | NM | 1 | S6 | NM | Hepatic splenosis | US-guided biopsy |
| 19 | 2008 | Yeh [25] | 64/M | Trauma | 8 | HCV | Asymptomatic | ALT, AST elevated | 1 | S6 | 2.5 | HCC | Surgery (liver resection) |
| 20 | 2009 | Hilal [26] | 60/M | Trauma | 46 | Cirrhosis | Flu-like symptoms, loss of weight, loss of appetite | LFT deranged, AFP elevated | Multiple | S7 | 2 × 2.5 and 4.5 | HCC | Explorative laparoscopy |
| 21 | 2009 | Kashgari [27] | 52/M | Trauma | 30 | HCV Cirrhosis | Asymptomatic | ALT, AST elevated | 1 | S7 | 2.1 × 1.5 | HCC | US-guided biopsy |
| 22 | 2009 | Menth [28] | 43/M | Trauma | 25 | HCV Cirrhosis | Asymptomatic | ALT, AST elevated | Multiple | S2 | 0.4-3.6 | HCC | Tc-99m DRBC |
| 23 | 2009 | Yu [29] | 54/M | Trauma | 20 | Nil | Asymptomatic | Normal | 1 | S2 | 4 | Uncertain | Surgery (liver resection) |
| 24 | 2010 | Mescoli [30] | 68/F | No splenectomy | NA | Cirrhosis | Abdominal pain | NM | Multiple | S3, S5, S7 | 6.2-11 | FNH/ haemangioma | Percutaneous biopsy |
| 25 | 54/M | Iatrogenic | 12 | Nil | Asymptomatic | NM | 1 | Left lobe | 3 | Liver metastasis | Surgery (explorative laparotomy) | ||
| 26 | 2010 | Tsitouridis [31] | 63/M | Trauma | 20 | Nil | RUQ pain | NM | 1 | Left lobe | 8 | Splenosis | CT-guided biopsy |
| 27 | 64/M | Gastric leiomyo-sarcoma | 1.5 | Nil | Asymptomatic | NM | 1 | NM | 5 | Peritoneal implantation | CT-guided biopsy | ||
| 28 | 2011 | Kang [32] | 54/M | Trauma | 15 | Nil | Asymptomatic | Normal | 2 | S2 | 0.7 × 0.6, 2.3 × 1.9 | Liver metastasis | Surgery (liver resection) |
| 29 | 2012 | Li [33] | 61/M | Trauma | NM | Nil | Asymptomatic | NM | Multiple | NM | NM | Hepatic splenosis | Needle biopsy |
| 30 | 2012 | Liu [7] | 38/M | Trauma | 14 | HBV | Asymptomatic | Normal | 1 | S2 | 3.3 × 2.7 | Liver tumour | Surgery (laparoscopic resection) |
| 31 | 2013 | Inchingolo [34] | 53/M | Trauma | 33 | NASH | Asymptomatic | GGT elevated | 1 | S3 | 3.5 | HCC/adenoma | Surgery (laparoscopic converted to open liver resection) |
| 32 | 2013 | Krawczyk [35] | 39/F | Trauma | NM | Nil | Abdominal pain | NM | 2 | S2, adjacent to major curvature of stomach | 3.2 × 2.0 | Adenoma | Tc-99m DRBC |
| 33 | 2013 | Leong [36] | 56/M | Trauma | NM | Nil | Chronic epigastric pain | NM | 1 | S3 | 3.7 × 4.6 × 3.1 | Carcinoid neuroendocrine tumour | Surgery (liver resection) |
| 34 | 2014 | Kandil [37] | 45/F | Haemolytic anaemia | 20 | HCV | Chronic abdominal pain | Normal | 1 | Left lobe | 5 × 4 | HCC | Surgery (explorative laparotomy) |
| 35 | 2014 | Sato [38] | 58/M | No splenectomy | NA | HCV Cirrhosis | Asymptomatic | ALT, AST, AFP elevated | 1 | Right lobe | 3.9 × 3 | HCC | Surgery (liver resection) |
| 36 | 2014 | Tinoco Gonzalez [39] | 60/M | Trauma | NM | HCV | Asymptomatic | NM | 1 | S3 | 4.8 | HCC/ Adenoma | Surgery (liver resection) |
| 37 | 2015 | Grambow [40] | 53/M | Trauma | 9 | Alcohol Cirrhosis | Incidental finding due to refractory ascites secondary to decompensated cirrhosis | Normal | 1 | S3, S4b | 3.5 | HCC | Surgery (laparotomy) |
| 38 | 2015 | Li [41] | 67/F | Trauma | 5 | HCV Cirrhosis | Asymptomatic | LFT deranged, AFP elevated | 1 | Left lobe | NM | HCC | Surgery (explorative laparotomy) |
| 39 | 2015 | Liu [6] | 33/M | Trauma | 30 | Nil | Asymptomatic | Normal | Multiple | Left and right lobes | 4.2 × 3.0 | HCC | FNA biopsy |
| 40 | 2015 | Tamm [42] | 43/M | Trauma | NM | Nil | RUQ pain | NM | 1 | S3 | 2.8 | Nil | Tc-99m DRBC |
| 41 | 2015 | Toktas [43] | 40/F | Idiopathic thrombocytopenic purpura | 7 | Nil | Asymptomatic, persistent low platelets | NM | 1 | S2/S3 | 7.0 × 3.0 | Nil | Surgery (liver resection) |
| 42 | 2015 | Wu [44] | 33/M | Trauma | 12 | Nil | Asymptomatic | Bilirubin elevated | 1 | S2 | 3.5 × 2.0 | HCC | Surgery (explorative laparotomy) |
| 43 | 2016 | Fung [45] | 55/M | Trauma | 37 | Nil | Asymptomatic | Normal | 2 | S6, S7 | 2.27 × 3.04 and 1.15 × 1.21 | Nil | Surgery (liver resection) |
| 44 | 2016 | Chen [46] | 51/M | Trauma | 20 | Nil | Asymptomatic | NM | 2 | Left and right lobes | 2.1; 3.3 × 2.6 | HCC | US-guided biopsy |
| 45 | 2016 | Jereb [47] | 22/M | Trauma | 18 | Nil | Asymptomatic | Normal | Multiple | S2, S6, S7 | 2.6 | Liver metastases | Surgery (explorative laparoscopy) |
| 46 | 2017 | Keck [48] | 66/M | NM | NM | Chronic HCV | Asymptomatic | Normal | Multiple | S7, S8 | 5.3 | Nil | Needle biopsy |
| 47 | 2017 | Somsap [49] | 51/M | Thalassemia | 20 | Nil | Abdominal pain | ALT, AST, bilirubin elevated | 1 | Left lobe | NM | HCC | Surgery (liver resection) |
| 48 | 2017 | Wang [5] | 54/M | Trauma | 23 | Chronic HBV | RUQ pain | Normal | 1 | Right lobe | 3.9 × 3.6 | HCC | Surgery (liver resection) |
| 49 | 2017 | Wang [50] | 42/M | Trauma | 16 | HBV, HCV, fatty liver | Chronic low back pain | Normal | 1 | S4 | 2.3 × 1.8 | HCC | Surgery (liver resection) |
| 50 | 2018 | Aramoana [51] | 58/M | Trauma | 37 | Nil | RUQ pain | Normal | 1 | S6 | 4.6 × 3.4 | HCC | Surgery (liver resection) |
| 51 | 2018 | Budak [52] | 46/M | Trauma | 30 | Nil | NM | NM | 2 | S6, S7 | 3.6 | HCC/hepatic splenosis | Tc-99m DRBC |
| 52 | 2018 | Guzman [53] | 43/M | Trauma | 16 | Nil | Acute RUQ pain | ALT, AST elevated | 1 | S2 | 2.5 | Adenoma | Percutaneous needle biopsy |
| 53 | 2018 | Smolen [54] | 35/M | Trauma | 12 | Nil | Chronic abdominal pain | Normal | Multiple | Left and right lobes | 4.3 | Adenoma/FNH | Tc-99m DRBC |
| 54 | 2018 | Teles [55] | 73/M | NM | NM | Nil | Low back pain | CEA elevated | Multiple | Left and right lobes, lumbar spine | 4.9 | Primary or secondary neoplasia | Surgery (open liver resection) |
| 55 | 2018 | Varghese [56] | 50/M | Trauma | 40 | Nil | Asymptomatic | NM | 1 | Right lobe, multiple extrahepatic nodules | 3.0 | Nil | Contrasted CT scan resembling splenic enhancement and clinical judgement |
| 56 | 2018 | Vergara [57] | 69/M | Trauma | NM | Nil | RUQ pain, dyspnoea, lower limb oedema | Normal | Multiple | S6, near falciform ligament, left para-vesical space | 6.5 × 4.6 | Nil | Needle biopsy |
| 57 | 2018 | Xuan [58] | 54/M | Trauma | 5 | Nil | Asymptomatic | Normal | 1 | S4 | 4.5 × 3.3 | HCC | Surgery (liver resection) |
| 58 | 2019 | Guedes [59] | 68/M | Trauma | 44 | Nil | Chronic epigastric and right hypochondrium pain | Normal | 1 | S6 | 3.0 | HCC/Adenoma | Surgery (laparoscopic liver resection) |
| 59 | 2019 | Luo [4] | 41/M | Trauma | 21 | Nil | Asymptomatic | 1 | Right lobe | NM | HCC | Surgery (explorative la |
AFP – α-fetoprotein, ALP – alkaline phosphatase, ALT – alanine aminotransferase, AST – aspartate aminotransferase, CT – computed tomography, F – female; FNA – fine needle aspiration, FNH – focal nodular hyperplasia, GGT – γ-glutamyltransferase, HCC – hepatocellular carcinoma, INR – international normalized ratio, LFT – liver function test, M – male, NA – not applicable, NM – not mentioned, RUQ – right upper quadrant, S1-S7 – segments I to VII of the liver, Tc-99m DRBC – technetium-99m-tagged heatdamaged red blood cell scan, US – ultrasound
Time (years) refers to the interval after splenectomy to discovery of intrahepatic splenosis
Laboratory investigations refer to basic liver function test and tumour marker (AFP). Hepatitis B and C serology is not included
Fig. 1.
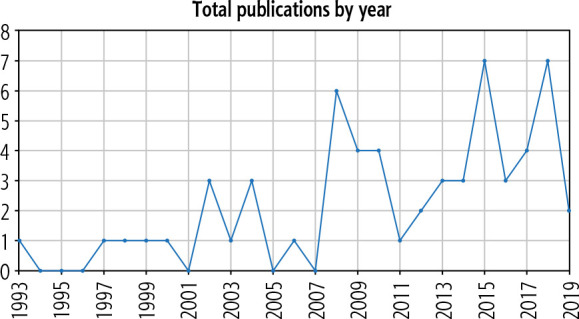
Pictorial representation showing the increasing trend of reporting of cases of intrahepatic splenosis
Results
Fifty-nine patients with IHS are reported with male predominance (n = 49, 83.1%) and a median age of 51 years (range 21-73 years). The majority of the patients had a prior history of splenectomy (n = 57, 95.0%). Two patients did not have any history of abdominal trauma or splenectomy. The median time from splenectomy to diagnosis of splenosis was 21 years (range 1.5-47 years). Reported risk factors for HCC were as follows: 1) hepatitis B (n = 8, 13.6%), 2) hepatitis C (n = 12, 20.3%), 3) heavy alcohol use (n = 2, 3.4%), 4) fatty liver (n = 3, 5.1%) and 5) cirrhosis (n = 12, 20.3%). 33 (55.9%) patients did not have any of the abovementioned risk factors for HCC. The majority of the patients were asymptomatic (n = 37, 62.7%). 19 patients (32.2%) presented with abdominal pain and/or discomfort and 3 patients (5.1%) had atypical presentations: 1 patient had flu-like symptoms, loss of weight and loss of appetite and 2 patients had chronic lower back pain.
Many of the reported cases do not include the essential laboratory investigations such as alanine aminotransferase (ALT), aspartate aminotransferase (AST) and α-fetoprotein (AFP). Of those cases which included these investigations, 12 out of 36 patients (33.3%) had transaminitis, and 6 out of 34 patients (17.6%) had raised AFP. The majority of the reported cases were isolated IHS; 4 (6.8%) of the cases included both intrahepatic and extrahepatic splenosis. The specific imaging features and patterns of enhancement can be found in the appendix (Table 2).
Table 2.
Patterns of enhancement on imaging of all cases (n = 59) of intrahepatic splenosis from 1939 to 2019
| No. | Year | Author | CT findings | MRI findings | Angiography |
|---|---|---|---|---|---|
| 1 | 1993 | Yoshimitsu [8] | Non-contrast: homogeneous low attenuation mass Contrast: enhanced from the periphery in the early phase, low attenuation in the delayed phase | T1-W: homogeneously low intensity T2-W: not obtained PDI: high intensity | Mass supplied by the left hepatic artery No definite neovascularity |
| 2 | 1997 | Gruen [9] | Contrast: high-attenuation mass | NA | NA |
| 3 | 1998 | D’Angelica [10] | Contrast: high-density mass | NA | NA |
| 4 | 1999 | Foroudi [11] | Contrast: multiple foci of enhancing soft tissue densities | NA | NA |
| 5 | 2000 | De Vuysere [12] | Non-contrast: slightly hypodense Contrast: homogeneously hyperdense in the arterial phase, isodense in the portal venous phase, and slightly hypodense in the late phase | Pre-contrast T1-W: hypointense Pre-contrast T2-W: hyperintense Post-contrast (small iron oxide particles (SPIO-Endorem): remained slightly hyperintense relative to the hypointense liver | NA |
| 6 | 2002 | Gamulin [13] | Contrast: heterogeneous enhancement | NA | NA |
| 7 | 2002 | Lee [14] | Contrast: early contrast enhancement and washout on delayed phase | NA | Tumour stained in segment 6 through the inferior phrenic artery No feeding vessel from hepatic or superior mesenteric artery |
| 8 | 2002 | Pekkafali [15] | Non-contrast: slightly hypodense with prominent hypodense rim around the lesion Contrast: hyperdense in the arterial phase, isodense in the portal venous phase and hypodense in the equilibrium phase | Pre-contrast T1-W: homogenously hypointense with hypointense rim Pre-contrast T2-W: isointense to liver with thin hypointense rim Post-contrast: hyperintense to liver | NA |
| 9 | 2003 | Kim [16] | Contrast: homogeneously well enhanced in the arterial phase and isodense in the equilibrium phase | NA | Mass supplied by inferior phrenic artery |
| 10 | 2004 | Di Costanzo [17] | Contrast: arterial hypervascularization and rapid “washout” of the contrast medium on portal venous phase | NA | NA |
| 11 | Contrast: early enhancement on the arterial phase and complete “washout” of the lesion on portal venous phase | NA | NA | ||
| 12 | 2004 | Kondo [18] | Contrast: low-density tumour in arterial phase, with vessels penetrating inside the tumour. Nearly homogeneous enhancement | T1-W: low signal intensity T2-W: high signal intensity | Hypervascular tumour supplied by the right hepatic artery |
| 13 | 2006 | Ferraioli [19] | NA | Contrast material-enhanced T1-W: liver tumour and accessory spleen were hypointense T2-W: liver tumour and accessory spleen were hyperintense | NA |
| 14 | 2008 | Choi [20] | Contrast: Lesion in segment IVa: slight enhancement during both the arterial and portal phase Lesion in segment VI: slight enhancement only in the portal phase | Contrast: enhancement during arterial phase and slightly hyperintense signal in the liver parenchyma during portal phase | Subtle tumour staining in segment IVa and no tumour staining in segment VI |
| 15 | 2008 | Grande [21] | Non-contrast: slightly hypodense compared to the liver Contrast: hyperdense in the arterial phase and isodense in the portal phase | NA | NA |
| 16 | 2008 | Imbriaco [22] | Non-contrast: hypodense Contrast: heterogeneous enhancement in the arterial phase, hypodense compared with the surrounding parenchyma during the portal and equilibrium phases | Pre-contrast T1-W: hypointense Pre-contrast T2-W: slightly hyperintense Post-contrast: nonhomogeneous enhancement during the arterial phase, hypointensity during the portal and equilibrium phases | |
| 17 | 2008 | Lu [23] | Non-contrast: two hypodense nodules Contrast: homogeneously hyperdense in the arterial phase, isodense in the portal venous phase, and slightly hypodense in the equilibrium phase. | Pre-contrast T1-W: homogeneously hypointense Pre-contrast T2-W: hyperintense contrast (Gd-DTPA): global enhancement in arterial phase, isointense in portal phase | |
| 18 | 2008 | Nakajima [24] | Non-contrast: hypodense mass Contrast: strong enhancement at the early phase and pooling enhancement at the late phase | T1-W: hypointense mass T2-W: hypointense mass |
|
| 19 | 2008 | Yeh [25] | Non-contrast: isodense Contrast: persistent homogeneous enhancement in the arterial and portal venous phases | Pre-contrast T2-W: intermediate to high signal Plain phase: iso-signal in the plain phase Post-contrast: heterogeneous enhancement in the arterial phase and persistent homogeneous enhancement in the portal venous phase | Tumour stain with blood supply via perirenal vessel |
| 21 | 2009 | Kashgari [27] | NA | Pre-contrast T1-W: mildly hypointense Pre-contrast T2-W: homogenously hyperintense Contrast (gadopentetate dimeglumine): heterogenous early arterial enhancement, isointense in porto-venous and equilibrium phase | NA |
| 20 | 2009 | Hilal [26] | Contrast: hypervascular nodule with increased enhancement in the venous phase | Contrast (gadolinium): hypervascular nodule in arterial and portal venous phase | NA |
| 22 | 2009 | Menth [28] | NA | Contrast (Gd-DTPA): marked enhancement in early arterial phase Contrast (SPIO) T2-W: lacks iron uptake | Regular branches of hepatic artery No pathologic vessels or parenchymal foci of hypervascularity |
| 23 | 2009 | Yu [29] | Contrast: strong and slightly inhomogeneous enhancement in the arterial phase, diminished enhancement in the portal venous phase | T1-W: hypointense T2-W: | |
| 24 | 2010 | Mescoli [30] | Contrast: hyper-enhancement in arterial and portal phases The largest nodule showed a hypodense central (necrotic) area | NA | NA |
| 25 | Contrast: hypervascular nodule | NA | NA | ||
| 26 | 2010 | Tsitouridis [31] | Non-contrast: slightly hypodense Contrast: increased enhancement during arterial phase with hypodense rim surrounding lesion. Lesion is isodense during portal phase | Pre-contrast T2-HASTE: intermediate-to-high signal intensity Post-contrast T2-HASTE: homogeneous enhancement with imaging characteristics of an extrahepatic-intraperitoneal lesion | NA |
| 27 | Contrast: hypodense with peripheral enhancement in both arterial and portal phases | Pre-contrast T2-HASTE: intermediate-to-high signal Post-contrast T2-HASTE: delayed peripheral enhancement Coronal plane: imaging characteristics of an extrahepatic lesion mimicking peritoneal implantation | NA | ||
| 28 | 2011 | Kang [32] | No parenchymal abnormality in liver | T1-W: low signal intensity T2-W: slightly high signal intensity slightly high signal intensity on the SPIO-enhanced T2-W: high signal intensity | NA |
| 29 | 2012 | Li [33] | Non-contrast: isodense masses mirroring residual spleen Contrast: enhancement in both hepatic mass and residual spleen | Pre-contrast T1-W: hypointense Pre-contrast T2-W: hyperintense Contrast: heterogeneous enhancement in arterial phase | NA |
| 30 | 2012 | Liu [7] | Non-contrast: homogeneous soft tissue mass with surrounding low-density aureole Contrast: slightly lower density than the liver especially in arterial phase | NA | NA |
| 31 | 2013 | Inchingolo [34] | Contrast: marked enhancement in arterial phase, remained hyperdense in portal venous phase | Post-contrast (gadolinium): increased arterialization after gadolinium injection with some loss of signal in the in-phase, indicating hemosiderin accumulation in the tissue DWI: restricted diffusion within the lesion | NA |
| 32 | 2013 | Krawczyk [35] | NI | Pre-contrast T2-W: hyperintense lesion in liver, with additional lesions dorsal to stomach that looks typical for regenerate spleen tissue Post-contrast T1-W: homogeneous enhancement | |
| 33 | 2013 | Leong [36] | Hypervascular lesion | Non-cystic irregular lesion with features suggestive of neuroendocrine tumour | |
| 34 | 2014 | Kandil [37] | Contrast: enhancement in arterial phase | NA | NA |
| 35 | 2014 | Sato [38] | Contrast: slightly inhomogeneous enhancement in arterial phase, with diminished enhancement in the equilibrium phase | Pre-contrast T2-W: hyperintense Post-contrast (Gd-EOB): hypointense compared to surrounding liver parenchyma | NA |
| 36 | 2014 | Tinoco | NA | Hypervascular lesion | NA |
| [39] | Contrast: homogeneous enhancement in arterial phase, with | ||||
| lavage in the portal phase and equilibrium | |||||
| 37 | 2015 | Grambow | Contrast: hypervascular mass with enhancement typical for HCC | NA | NA |
| [40] | |||||
| 38 | 2015 | Li [41] | Contrast: strong homogeneous enhancement in arterial phase and | Pre-contrast T1-W: slightly hyperintense | Hypervascular tumour supplied by the |
| hypodense during portal phase | Pre-contrast T2-W: slightly hyperintense | branches of the hepatic artery | |||
| Post-contrast T2-W: hyperintense during arterial phase and | |||||
| hypointense during the portal phase | |||||
| 39 | 2015 | Liu [6] | NI | T2-W: intermediate-to-high signal intensity | NA |
| 40 | 2015 | Tamm | Non-contrast: slightly hypodense | Pre-contrast T1-W: hypointense | |
| [42] | Contrast: hypodense during arterial phase and hyperdense during | Pre-contrast T2-W: mildly hyperintense | |||
| portal venous phase | Post-contrast: no brisk arterial enhancement was present after | ||||
| contrast administration. Presence of homogeneous enhancement | |||||
| at 1 minute, with central washout and a residual rim of peripheral | |||||
| enhancement at 5 minutes | |||||
| 41 | 2015 | Toktas [43] | Isodense with spleen | NA | NA |
| 42 | 2015 | Wu [44] | Non-contrast: homogeneous hypodense mass | T1-W: low signal intensity | NA |
| T2-W: high signal intensity | |||||
| 43 | 2016 | Fung [45] | Contrast: early arterial enhancement with | Pre-contrast T1-W: hypointense | NA |
| contrast washout in delayed phase | Pre-contrast T2-W: hyperintense | ||||
| Post-contrast T2-W: enhancement in arterial phase followed by | |||||
| washout in delayed phase | |||||
| 44 | 2016 | Chen [46] | Contrast: marked | Pre-contrast T1-W: low signal intensity | NA |
| enhancement at arterial phase and delayed phase | Post-contrast T1-W: lower enhancement after contrast | ||||
| administration | |||||
| 45 | 2016 | Jereb [47] | Contrast: hypodense lesions in portal phase | Post-contrast T1-W: hypointense in both arterial and late phase | NA |
| Post-contrast T2-W: hyperintense during arterial phase, | |||||
| hypointense in late phase | |||||
| 46 | 2017 | Keck [48] | NA | Arterial enhancement with washout | NA |
| 47 | 2017 | Somsap | NA | Pre-contrast T1-W: hypointense | NA |
| [49] | Post-contrast T1-W: heterogenous enhancement during arterial | ||||
| phase, more homogeneous in portal and delayed phase | |||||
| 48 | 2017 | Wang [5] | Non-contrast: hypodense | Pre-contrast T1-W: slightly hypointense | NA |
| Contrast: strong homogeneous enhancement in arterial phase and | Pre-contrast T2-W and DWI: high signal intensity | ||||
| hypodense during portal phase | Post-contrast T2-W: uneven enhancement with decreased signal | ||||
| 49 | 2017 | Wang [50] | Contrast: marked homogeneous enhancement in arterial and portal | Pre-contrast T1-W: hypointense | NA |
| venous phase, with diminished enhancement in the equilibrium phase | Pre-contrast T2-W: hyperintense | ||||
| Post-contrast: moderate homogeneous enhancement with marked | |||||
| delayed ring enhancement mimicking a pseudocapsule similar to | |||||
| hepatocellular carcinoma (HCC) in equilibrium phase | |||||
| 50 | 2018 | Aramoana | Contrast: enhancement in arterial phase | Post-contrast T2-W: peak enhancement at 60 s and washout | NA |
| [51] | at 10 min | ||||
| 51 | 2018 | Budak | NA | T2-HASTE: hyperintense | NA |
| [52] | Post-contrast T1-W: hepatic lesion showed marked enhancement | ||||
| in arterial phase. Multiple nodule formations in peritoneal cavity | |||||
| similarly showed similar contrast uptake pattern | |||||
| 52 | 2018 | Guzman | NI | NI | NA |
| [53] | |||||
| 53 | 2018 | Smolen | Non-contrast: multiple isodense lesions | NA | NA |
| [54] | Contrast: hyperenhancement in arterial phase, iso- to | ||||
| hypoenhancement in portal and delayed phase) | |||||
| Carcinoma could not be ruled out | |||||
| 54 | 2018 | Teles [55] | NI | NI | NA |
| 55 | 2018 | Varghese | Contrast: heterogeneous “arciform” enhancement in arterial phase, | NA | NA |
| [56] | with continued homogeneous enhancement in delayed phase with | ||||
| slow washout | |||||
| 56 | 2018 | Vergara | Contrast: mild enhancement in arterial phase | Pre-contrast T1-W: low signal intensity | NA |
| [57] | Pre-contrast T2-W: slightly hyperintense | ||||
| Post-contrast T1-W: lower enhancement compared surrounding | |||||
| liver parenchyma | |||||
| 57 | 2018 | Xuan [58] | Non-contrast: slightly hypodense | Pre-contrast T1-W and T2-W: slightly hypointense | NA |
| Contrast: inhomogeneous enhancement during arterial phase and | DWI: slightly hyperintense | ||||
| diminished enhancement during the portal and equilibrium phase | Post-contrast: strongly heterogeneous and hyperintense during | ||||
| the arterial phase and relatively hypointense during the portal | |||||
| 58 | 2019 | Guedes | NA | Pre-contrast T1-W: hypointense | NA |
| [59] | Pre-contrast T2-W: hyperintense | ||||
| Post-contrast: increased vascularity and washed out during late | |||||
| venous phase | |||||
| 59 | 2019 | Luo [4] | Non-contrast: multiple hypodense lesions | NA | NA |
| Contrast: enhancement during arterial phase with hypodense rim |
CT – computed tomography, DWI – diffusion-weighted imaging, Gd-DTPA – gadolinium-diethylenetriaminepentaacetic acid, Gd-EOB – gadoxetic acid, HCC – hepatocellular carcinoma, MRI – magnetic resonance imaging, PDI – proton density image,
SPIO – superparamagnetic iron oxide, T1-W – T1-weighted, T2-W – T2-weighted
NA – not applicable, NI – no information on enhancement pattern
HCC was considered the initial diagnosis in 29 patients (49.2%). IHS was considered as the primary diagnosis in 9 patients (15.3%). There were several reported modalities for confirmatory diagnoses: open liver resection (n = 21, 35.6%), laparoscopic liver resection (n = 2, 3.4%), explorative laparotomy (n = 7, 18.9%), explorative laparoscopy (n = 3, 5.1%), percutaneous needle biopsy (n = 15, 25.4%), and Tc-99m denucleated RBC scintigraphy (n = 10, 16.9%). One patient (1.7%) only had the contrasted CT scan resembling splenic enhancement and was diagnosed with IHS based on the clinical history of splenectomy and absence of risk factors for HCC [56].
Discussion
Splenosis is an acquired condition and is defined as the autotransplantation of splenic tissue following abdominal or splenic trauma or splenectomy, displacing fragmented splenic tissues which may subsequently regrow at implanted sites by acquiring a vascular supply. It has been suggested that local hypoxia induced by hepatic diseases and/or aging may induce splenic erythropoiesis of previously seeded tissues [60]. This is in contrast to an accessory spleen, which is a congenital condition due to the failure of embryological fusion of the splenic primordium and arises from the left side of the dorsal mesogastrium [2, 38].
The major dilemma in the diagnosis of IHS is the need for exclusion of malignancy such as HCC or liver metastases. Radiological findings for IHS mimic the hallmarks of HCC: hyperenhancement in the arterial phase with delayed washout in the portal venous phase and low signal intensity in the hepatobiliary phase [61]. In the presence of risk factors such as hepatitis B, hepatitis C, heavy alcohol use and/or cirrhosis, primary liver malignancy such as HCC should always be excluded. Our study shows that the majority of the patients present with incidental liver lesions and do not have risk factors for HCC. In this group of patients, IHS should be considered and non-invasive or minimally invasive methods of confirmatory diagnosis should be explored. A non-invasive method to confirm the diagnosis of splenosis is the use of Tc-99m heat-damaged RBC scintigraphy [9]. This involves in vitro labelling of the patient’s RBC with Tc-99m, heating the RBC at 49ºC for 20 minutes, and subsequently injecting the patient with the Tc-99m labelled heatdamaged RBC and imaging with planar and singlephoton emission computed tomography (SPECT) 30 minutes later [62]. Splenic tissues will phagocytose the heat-damaged RBCs, enabling radioisotope uptake of Tc-99m labelled RBCs. This is a specific and relatively sensitive method of diagnosis of splenosis as compared to the use of sulfur colloid, as the spleen takes up more than 90% of heat-damaged RBC as compared to 10% of sulfur colloid [42, 63]. However, improper preparation of heat-damaged RBCs such as overheating or underheating may result in false negatives [64]. In addition, scintigraphy has poor anatomic localization, which warrants the need to correlate the lesions with higher definition scans such as magnetic resonance imaging (MRI). Our study shows that Tc-99m labelled heat-damaged RBC is not widely used to diagnose IHS. This could be due to its limited availability or cost. Another clue suggestive for IHS is the absence or decreased number of Howell-Jolly bodies seen in peripheral blood smears, which would be normally seen in patients with asplenia [65].
In addition, though radiological findings for splenosis may mimic other hepatic lesions, Tsitouridis et al. described the characteristic imaging of IHS on CT and MRI imaging: hypodense lesion on non-contrast CT. Following contrast administration, the lesion is hyperdense in the arterial phase, isodense in the portal venous phase and hypodense in the delayed phase [31]. MRI findings include homogeneous hypointensity and hyperintensity prior to contrast administration on T1-weighted and T2-weighted images respectively, with a characteristic hypointense rim surrounding the lesion on T1-weighted imaging [31]. In addition, demonstration of classic heterogenous or arciform enhancement in the arterial phase with homogeneous enhancement in the delayed phase is classic for splenic enhancement and may suggest HIS [56]. Based on available data, the diagnosis of IHS can be made based on the ‘triad’ of 1) history of splenectomy or abdominal trauma, 2) absence of risk factors for liver malignancy and 3) typical imaging pattern on contrast enhanced imaging. Considering this ‘triad’ as a diagnostic hallmark of IHS, sensitivity of this triad in all the 59 reported cases was: 96.6% (n = 57/59) for one or more features, 52.5% (n = 31/59) for two or more features and 5.1% (n = 3/59) for all three features. Undoubtedly, the presence of all three cardinal features is rare, but is likely able to confirm the diagnosis of IHS without the need for surgical resection. We were unable to analyse the specificity of this triad as all the cases reported are diagnosed to be IHS.
Other imaging modalities such as the use of contrast-enhanced ultrasound can exclude HCC. On contrast-enhanced ultrasound, HCC appears as homogeneous and hyperechoic compared with the surrounding liver tissue after contrast administration, with a rapid washout and becoming a hypoechoic lesion in the portal and sinusoidal phases [19]. Superparamagnetic iron oxide (SPIO) administration in MRI scans can aid in tissue characterization. SPIO is taken up by the reticuloendothelial cells of the liver and spleen and has been shown to improve the detection rate of benign hepatocellular tumours [66]. IHS will demonstrate hypointensity on T2-weighted MRI due to phagocytosis of iron particles by splenic reticuloendothelial cells. Abdominal imaging does have its limitations and may not provide a definite diagnosis. Absolute diagnosis as with any malignant lesion is possible by sampling the tissue. Percutaneous image-guided needle biopsy can establish a definite diagnosis by demonstrating normal splenic tissue with red pulp and white pulp, lymphocyte B cells and CD3-positive lymphocyte T cells [27]. The use of fine needle aspiration cytology has been previously reported to avoid unnecessary surgery [67]. However, results may be inconclusive, and patients may have to bear additional costs of further diagnostic tests.
Surgical resection should be reserved for patients with inconclusive imaging scans or biopsy findings, abdominal symptoms not attributed to any other pathology, those in whom malignancy cannot be ruled out with certainty, or those with presence of risk factors for HCC. Explorative laparoscopy with intraoperative frozen section could be considered to reduce morbidity following liver resection [7, 26]. Should patients be diagnosed with IHS using non-invasive or minimally invasive methods, surgery can be avoided if patients are asymptomatic [57]. It has been reported that the average interval from trauma and abdominal splenosis is 10 years (range from 5 months to 32 years) [68, 69]. This is in contrast to our review, which demonstrated a median time of 21 years (range 1.5-47 years) from splenectomy to diagnosis of splenosis. Nevertheless, splenosis should still be considered in patients with a history of splenectomy regardless of the time from splenectomy. There have been two reported cases of IHS without any history of abdominal trauma or splenectomy: a 68-year-old woman presenting with recurrent abdominal pain [30]; and an asymptomatic 58-year-old man presenting with work-up for transaminitis [38]. There is no explanation for this phenomenon, but these occurrences are rare and IHS should only be a diagnosis of exclusion in the absence of prior history of abdominal trauma or splenectomy.
In conclusion, this review summarizes the available body of evidence for IHS. We also report a diagnostic triad: 1) history of splenectomy or abdominal trauma, 2) absence of risk factors for liver malignancy and 3) typical imaging features on contrast-enhanced imaging. In the presence of risk factors for HCC, malignancy should be ruled out. Non-invasive diagnostic tests such as Tc-99m heat-damaged RBC scintigraphy are useful in diagnosis. Surgery is reserved for patients with (1) abdominal pain or other symptoms which cannot be attributed to pathology or (2) inability to rule out malignancy. Clinicians should be aware of this rare pathology and all cases should be reported to enhance the knowledge and understanding of this disease.
Disclosure
The authors declare no conflict of interest.
References
- 1.Buchbinder J, Lipkoff C. Splenosis: multiple peritoneal splenic implants following abdominal injury: a report of a case and review of the literature. Surgery 1939; 6: 927-934. [Google Scholar]
- 2.Fremont RD, Rice TW. Splenosis: a review. South Med J 2007; 100: 589-594. [DOI] [PubMed] [Google Scholar]
- 3.Ksiadzyna D, Pena A. Abdominal splenosis. Revista Espańola de Enfermedades Digestivas. 2011; 103: 421-426. [PubMed] [Google Scholar]
- 4.Luo X, Zeng J, Wang Y, et al. . Hepatic splenosis: Rare yet important–A case report and literature review. J Int Med Res 2019; 47: 1793-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W-C, Li X-F, Yan Z-L, et al. . Intrahepatic splenosis mimics hepatocellular carcinoma in a patient with chronic hepatitis B: a case report and literature review. Medicine 2017; 96: e8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, Liu J, Wang F. Intrahepatic splenosis mimicking liver cancer: report of a case and review of literature. Int J Clin Exp Pathol 2015; 8: 1031-1035. [PMC free article] [PubMed] [Google Scholar]
- 7.Liu K, Liang Y, Liang X, et al. . Laparoscopic resection of isolated hepatic splenosis mimicking liver tumors: case report with a literature review. Surg Laparosc Endosc Percutan Tech 2012; 22: e307-311. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimitsu K, Aibe H, Nobe T, et al. . Intrahepatic splenosis mimicking a liver tumor. Abdom Imaging 1993; 18: 156-158. [DOI] [PubMed] [Google Scholar]
- 9.Gruen DR, Gollub M. Intrahepatic splenosis mimicking hepatic adenoma. AJR Am J Roentgenol 1997; 168: 725-726. [DOI] [PubMed] [Google Scholar]
- 10.D’Angelica M, Fong Y, Blumgart LH. Isolated hepatic splenosis: first reported case. HPB Surgery 1998; 11: 39-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foroudi F, Ahern V, Peduto A. Splenosis mimicking metastases from breast carcinoma. Clin Oncol 1999; 11: 190-192. [DOI] [PubMed] [Google Scholar]
- 12.De Vuysere S, Van Steenbergen W, Aerts R, et al. . Intrahepatic splenosis: imaging features. Abdom Imaging 2000; 25: 187-189. [DOI] [PubMed] [Google Scholar]
- 13.Gamulin A, Oberholzer J, Rubbia-Brandt L, et al. . An unusual, presumably hepatic mass. Lancet 2002; 360: 2066. [DOI] [PubMed] [Google Scholar]
- 14.Lee JB, Ryu KW, Song TJ, et al. . Hepatic splenosis diagnosed as hepatocellular carcinoma: report of a case. Surg Today 2002; 32: 180-182. [DOI] [PubMed] [Google Scholar]
- 15.Pekkafalı Z, Karslı FA, Şilit E, et al. . Intrahepatic splenosis: a case report. Eur Radiol 2002; 12 Suppl 3: S62-65. [DOI] [PubMed] [Google Scholar]
- 16.Kim KA, Park CM, Kim CH, et al. . An interesting hepatic mass: splenosis mimicking a hepatocellular carcinoma (2003: 9b). Eur Radiol 2003; 13: 2713-2715. [DOI] [PubMed] [Google Scholar]
- 17.Di Costanzo GG, Picciotto FP, Marsilia GM, et al. . Hepatic splenosis misinterpreted as hepatocellular carcinoma in cirrhotic patients referred for liver transplantation: report of two cases. Liver Transpl 2004; 10: 706-709. [DOI] [PubMed] [Google Scholar]
- 18.Kondo M, Okazaki H, Takai K, et al. . Intrahepatic splenosis in a patient with chronic hepatitis C. J Gastroenterol 2004; 39: 1013-1015. [DOI] [PubMed] [Google Scholar]
- 19.Ferraioli G, Di Sarno A, Coppola C, et al. . Contrast-enhanced low-mechanical-index ultrasonosraphy in hepatic splenosis. J Ultrasound Med 2006; 25: 133-136. [DOI] [PubMed] [Google Scholar]
- 20.Choi GH, Ju MK, Kim JY, et al. . Hepatic splenosis preoperatively diagnosed as hepatocellular carcinoma in a patient with chronic hepatitis B: a case report. J Korean Med Sci 2008; 23: 336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grande M, Lapecorella M, Ianora AAS, et al. . Intrahepatic and widely distributed intraabdominal splenosis: multidetector CT, US and scintigraphic findings. Intern Emerg Med 2008; 3: 265-267. [DOI] [PubMed] [Google Scholar]
- 22.Imbriaco M, Camera L, Manciuria A, et al. . A case of multiple intra-abdominal splenosis with computed tomography and magnetic resonance imaging correlative findings. World J Gastroenterol 2008; 14: 1453-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu HC, Su CW, Lu CL, et al. . Hepatic splenosis diagnosed by spleen scintigraphy. Am J Gastroenterol 2008; 103: 1842-1844. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima T, Fujiwara A, Yamaguchi M, et al. . Intrahepatic splenosis with severe iron deposition presenting with atypical magnetic resonance images. Intern Med 2008; 47: 743-746. [DOI] [PubMed] [Google Scholar]
- 25.Yeh ML, Wang LY, Huang CI, et al. . Abdominal splenosis mimicking hepatic tumor: a case report. Kaohsiung J Med Sci 2008; 24: 602-606. [DOI] [PubMed] [Google Scholar]
- 26.Hilal MA, Harb A, Zeidan B, et al. . Hepatic splenosis mimicking HCC in a patient with hepatitis C liver cirrhosis and mildly raised alpha feto protein; the important role of explorative laparoscopy. World J Surg Oncol 2009; 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashgari AA, Al-Mana HM, Al-Kadhi YA. Intrahepatic splenosis mimicking hepatocellular carcinoma in a cirrhotic liver. Saudi Med J 2009; 30: 429-432. [PubMed] [Google Scholar]
- 28.Menth M, Herrmann K, Haug A, et al. . Intra-hepatic splenosis as an unexpected cause of a focal liver lesion in a patient with hepatitis C and liver cirrhosis: a case report. Cases J 2009; 2: 8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu H, Xia L, Li T, et al. . Intrahepatic splenosis mimicking hepatoma. BMJ Case Rep 2009; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mescoli C, Castoro C, Sergio A, et al. . Hepatic spleen nodules (HSN). Scand J Gastroenterol 2010; 45: 628-632. [DOI] [PubMed] [Google Scholar]
- 31.Tsitouridis I, Michaelides M, Sotiriadis C, et al. . CT and MRI of intraperitoneal splenosis. Diagn Interv Radiol 2010; 16: 145-149. [DOI] [PubMed] [Google Scholar]
- 32.Kang KC, Cho GS, Chung GA, et al. . Intrahepatic splenosis mimicking liver metastasis in a patient with gastric cancer. J Gastric Cancer 2011; 11: 64-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Snow-Lisy D, Klein EA. Hepatic splenosis diagnosed after inappropriate metastatic evaluation in patient with low-risk prostate cancer. Urology 2012; 79: e73-74. [DOI] [PubMed] [Google Scholar]
- 34.Inchingolo R, Peddu P, Karani J. Hepatic splenosis presenting as arterialised liver lesion in a patient with NASH. Eur Rev Med Pharmacol Sci 2013; 17: 2853-2856. [PubMed] [Google Scholar]
- 35.Krawczyk M, Schneider G, Farmakis G, et al. . Splenosis mimicking hepatic adenoma. J Clin Exp Hepatol 2013; 3: 351-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leong CW, Menon T, Rao S. Post-traumatic intrahepatic splenosis mimicking a neuroendocrine tumour. BMJ Case Rep 2013; 2013: bcr2012007885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kandil TS, Kandil TS, El Sorogy M, et al. . Post-splenectomy splenosis presenting as hepatocellular carcinoma in the left lateral section of the liver: A case report. Int J Surg Case Rep 2014; 5: 877-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato N, Abe T, Suzuki N, et al. . Intrahepatic splenosis in a chronic hepatitis C patient with no history of splenic trauma mimicking hepatocellular carcinoma. Am J Case Rep 2014; 15: 416-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tinoco González J, Suárez Artacho G, Ramallo Solís IM, et al. . Intrahepatic splenosis as a differential diagnosis in focal liver lesions. Cir Esp 2014; 92: 690-691. [DOI] [PubMed] [Google Scholar]
- 40.Grambow E, Weinrich M, Zimpfer A, et al. . Ectopic spleen tissue–an underestimated differential diagnosis of a hypervascularised liver tumour. Viszeralmedizin 2015; 31: 445-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li T, Yang XY, Tang ZY. Intrahepatic and intraperitoneal splenosis mimicking hepatocellular carcinoma with abdominal wall metastasis in a patient with hepatitis C cirrhotic liver. Surgery 2015; 157: 954-956. [DOI] [PubMed] [Google Scholar]
- 42.Tamm A, Decker M, Hoskinson M, et al. . Heat-damaged RBC scan: a case of intrahepatic splenosis. Clin Nucl Med 2015; 40: 453-454. [DOI] [PubMed] [Google Scholar]
- 43.Toktaş O, Yavuz A, İliklerden Ü, et al. . Intrahepatic splenosis after splenectomy performed for idiopathic thrombocytopenic purpura. Ulus Cerrahi Derg 2015; 31: 247-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu C, Zhang B, Chen L, et al. . Solitary perihepatic splenosis mimicking liver lesion: a case report and literature review. Medicine 2015; 94: e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fung A, Chok K, Lo A, et al. . Hepatobiliary and pancreatic: hepatic splenosis: a rare differential of a liver mass in an HBV endemic area. J Gastroenterol Hepatol 2016; 31: 1238. [DOI] [PubMed] [Google Scholar]
- 46.Chen H, Wang X, Heng PA, et al. . Automated mitosis detection with deep regression networks. 2016, IEEE 13th International Symposium on Biomedical Imaging. IEEE International Symposium on Biomedical Imaging 2016; 1204-1207. [Google Scholar]
- 47.Jereb S, Trotovsek B, Skrbinc B. Hepatic splenosis mimicking liver metastases in a patient with history of childhood immature teratoma. Radiol Oncol 2016; 50: 212-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keck C, Shetty A, Mischen B, et al. . Hepatic splenosis masquerading as hepatocellular carcinoma in a chronic hepatitis C patient. Am J Gastroenterol 2017; 112: 1493. [DOI] [PubMed] [Google Scholar]
- 49.Somsap K, Chamadol N, Titapun A, et al. . MR imaging findings of a patient with isolated intrahepatic splenosis mistaken for hepatocellular carcinoma. BJR Case Rep 2017; 3: 20150242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang MY, Li B, Chen D, et al. . Spleen implanting in the fatty liver mimicking hepatocarcinoma in a patient with hepatitis B&C: A case report and literature review. Medicine 2017; 96: e7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aramoana J, Wylie N, Koea JB. Hepatic splenosis. ANZ J Surg 2018; 88: E359-360. [DOI] [PubMed] [Google Scholar]
- 52.Budak E, Oral A, Yazici B, et al. . Splenosis of the liver capsule. Clin Nucl Med 2018; 43: e460-462. [DOI] [PubMed] [Google Scholar]
- 53.Guzman Rojas P, Parikh J, Mahne A, et al. . Where is my spleen? –a case of splenosis diagnosed years later after splenectomy. Cureus 2018; 10: e2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smolen B, Khoury J, Baruch Y, et al. . Non-invasive evaluation of a liver mass in a patient post splenectomy. Scott Med J 2019; 64: 35-39. [DOI] [PubMed] [Google Scholar]
- 55.Teles GNS, Monteiro PEZ, Raphe R. Intrahepatic splenosis mimicking hepatic neoplasia. Int J Surg Case Rep 2018; 44: 47-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varghese J, Bergson J, Yaipen O. Intrahepatic splenosis: incidental liver lesion after splenectomy. J Comput Assist Tomogr 2018; 42: 730-731. [DOI] [PubMed] [Google Scholar]
- 57.Vergara D, Ginolfi F, Moscati S, et al. . Multiple intra-hepatic and abdominal splenosis: an easy call if you know about it. Acta Radiol Open 2018; 7: 2058460118772324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xuan Z, Chen J, Song P, et al. . Management of intrahepatic splenosis:a case report and review of the literature. World J Surg Oncol 2018; 16: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guedes TP, Fernandes B, Pedroto I. Hepatobiliary and pancreatic: symptomatic hepatic splenosis. J Gastroenterol Hepatol 2019; 34: 2061. [DOI] [PubMed] [Google Scholar]
- 60.Kwok CM, Chen YT, Lin HT, et al. . Portal vein entrance of splenic erythrocytic progenitor cells and local hypoxia of liver, two events cause intrahepatic splenosis. Med Hypotheses 2006; 67: 1330-1332. [DOI] [PubMed] [Google Scholar]
- 61.Park YS, Lee CH, Kim JW, et al. . Differentiation of hepatocellular carcinoma from its various mimickers in liver magnetic resonance imaging: What are the tips when using hepatocyte-specific agents? World J Gastroenterol 2016; 22: 284-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MacDonald A, Burrell S. Infrequently performed studies in nuclear medicine: part 1. J Nucl Med Technol 2008; 36: 132-143. [DOI] [PubMed] [Google Scholar]
- 63.Hagan I, Hopkins R, Lyburn I. Superior demonstration of splenosis by heat-denatured Tc-99m red blood cell scintigraphy compared with Tc-99m sulfur colloid scintigraphy. Clin Nucl Med 2006; 31: 463-466. [DOI] [PubMed] [Google Scholar]
- 64.Jolepalem P, Balon HR. Application of heat-damaged Tc-99m RBCs in a patient with suspected hepatic metastasis. Radiol Case Rep 2013; 8: 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schenkein D, Ahmed E. Case 29-1995: a 65-year-old man with mediastinal Hodgkin’s disease and a pelvic mass. N Engl J Med 1995; 333: 784-791. [DOI] [PubMed] [Google Scholar]
- 66.Grandin C, Van BB, Robert A, et al. . Benign hepatocellular tumors: MRI after superparamagnetic iron oxide administration. J Comput Assist Tomogr 1995; 19: 412-418. [PubMed] [Google Scholar]
- 67.Galloro P, Marsilia G, Nappi O. Hepatic splenosis diagnosed by fine-needle cytology. Pathologica. 2003; 95: 57-59. [PubMed] [Google Scholar]
- 68.Fleming CR, Dickson ER, Harrison EG Jr. Splenosis: autotransplantation of splenic tissue. Am J Med 1976; 61: 414-419. [DOI] [PubMed] [Google Scholar]
- 69.Berman AJ, Zahalsky MP, Okon SA, et al. . Distinguishing splenosis from renal masses using ferumoxide-enhanced magnetic resonance imaging. Urology 2003; 62: 748. [DOI] [PubMed] [Google Scholar]


