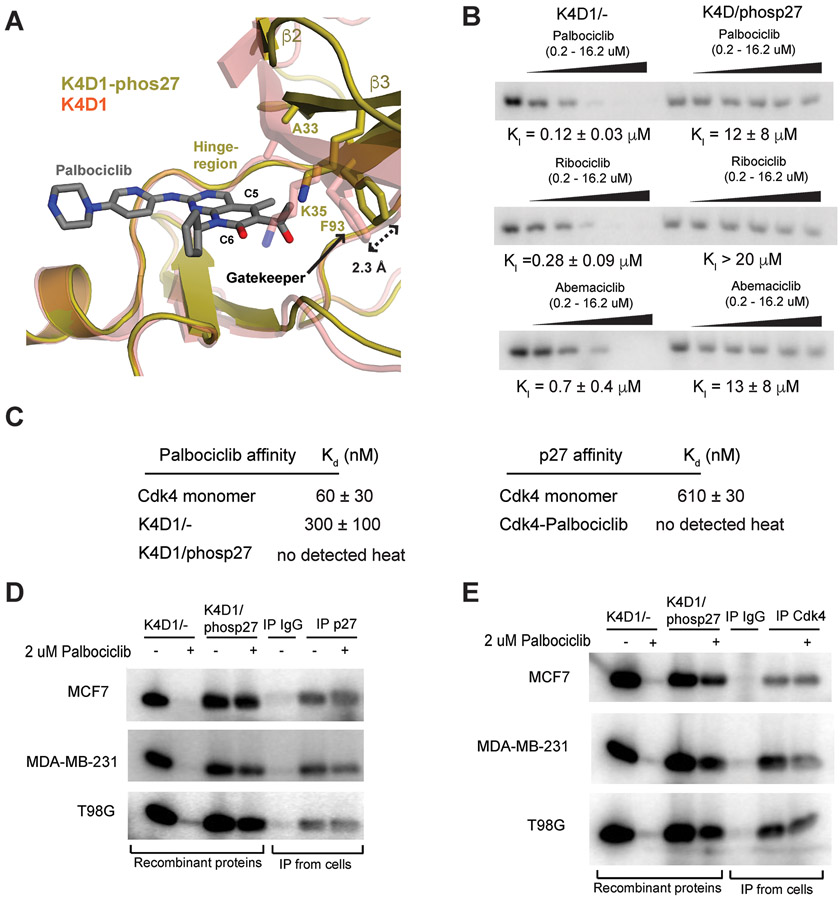
Fig. 5: Palbociclib does not bind and poorly inhibits purified and endogenous CDK4 trimer complexes.
(A) K4D1 dimer (PDB ID: 2W96) and the phosp27-K4D1 trimer structures were aligned with palbociclib-bound CDK6 (PDB ID: 2EUF, not shown) to model the position and interactions of the drug when bound to CDK4. (B) 32P-ATP phosphorylation of Rb771-928 using CDK4-CycD1 dimer (K4D1/−) and phosp27-CDK4-CycD1 trimer (K4D1/phosp27) enzymes in the absence (left most lane in each titration) and presence of increasing inhibitor concentrations (see Fig. S8A for quantification). Each drug is dosed from 0.2 μM to 16.2 μM in 3-fold increments. (C) ITC affinities for palbociclib (left) or p27 (right) titrated into the indicated enzyme. (D) The indicated cell lysates were immunoprecipitated with control or with p27 antibody, and the activity of the immunoprecipitate was used to phosphorylate Rb771-928 with 32P-ATP in the absence or presence of palbociclib. Reactions with the indicated recombinant dimer (K4D1/−) or trimer (K4D1/phosp27) enzymes are shown for comparison in the first four lanes. (E) As in panel D, except lysates were precipitated with antiserum raised against a CDK4 C-terminal peptide.