Figure 8.
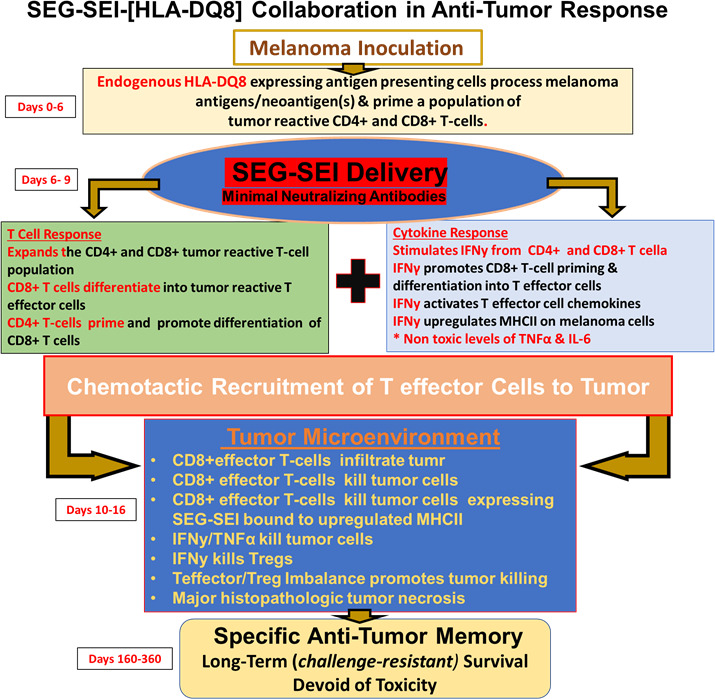
Antitumor effect and network induced by SEG/SEI in HLA-DQ8 tg mice resulting in long-term antitumor memory and melanoma survival is shown. Following melanoma inoculation on day 0, HLA-DQ8 expressing antigen presenting cells (APCs) recognize melanoma neoantigens and present them to T cells resulting in priming of tumor reactive CD4+ and CD8+T cells. This priming alone is insufficient to induce an anti-tumor response. Introduction of SEG/SEI on days 6 and 9, however, results in expansion of both CD4+ and CD8+T cells. Following SEG/SEI treatment the primed CD4 +T cell population likely d promotes CD8 +T cell differentiation into T effector cells. The superantigenic T cell activation also generates T cell chemotactic molecules that enables recruitment of T effector cells to the tumor. SEG/SEI further initiates a surge of IFNγ from both CD4 +and CD8+T cells. By days 13–16, the TME is infiltrated by CD8 +T effector cells along with IFNγ and TNFα that appear to constitute the major tumoricidal effectors. CD8 +T effector cell tumor killing is likely enhanced by the relative paucity of Tregs and myeloid cells in the TME. Long-term surviving SEG/SEI-treated mice exhibit specific anti-tumor memory by resisting late challenge with melanoma cells while succumbing to inoculation with Lewis lung carcinoma. The long-term antitumor response suggests a self-sustaining feed forward mechanism possibly mediated by persistence of SEI in vivo in a high affinity, Zn++ dependent interaction with the HLA-DQ8 allele. IFNγ, interferon-γ; IL-6, interleukin 6; TNFα, tumor necrosis factor-α.
