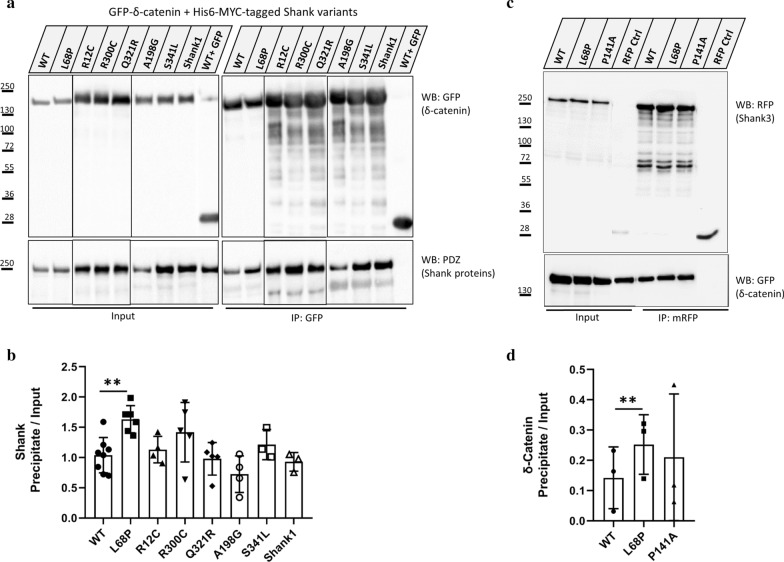
Fig. 4.
Effect of Shank3 N-terminal variants on binding to δ-catenin. a Shank3 WT and variants carrying ASD mutations, as well as Shank1, were coexpressed in 293 cells with GFP-tagged δ-catenin. Cell lysates were subjected to coimmunoprecipitation of GFP-δ-catenin using GFP-trap. b Quantification of the results in A, shown. as the ratio of Shank3 IP/input signals. The L68P mutation significantly increases the binding of δ-catenin to Shank3. Shank1 is also able to interact with δ-catenin via its N-terminal domain (n = 4, one-way ANOVA with Dunnett’s Test, **p < 0.01, mean ± SD). c δ-catenin was coexpressed with RFP-tagged Shank3 WT or variants carrying L68P and P141A mutations; cells were lysed and lysates were subjected to immunoprecipitation using RFP-trap. d Quantification of the results in c, shown as the ratio of GFP-δ-catenin IP/input signals. While the effect of L68P is again significant in improving binding of δ-catenin to Shank3, the de novo mutation P141A slightly (but non-significantly) improves the binding (n = 3, one-way ANOVA with Dunnett’s Test, **p < 0.01, mean ± SD)