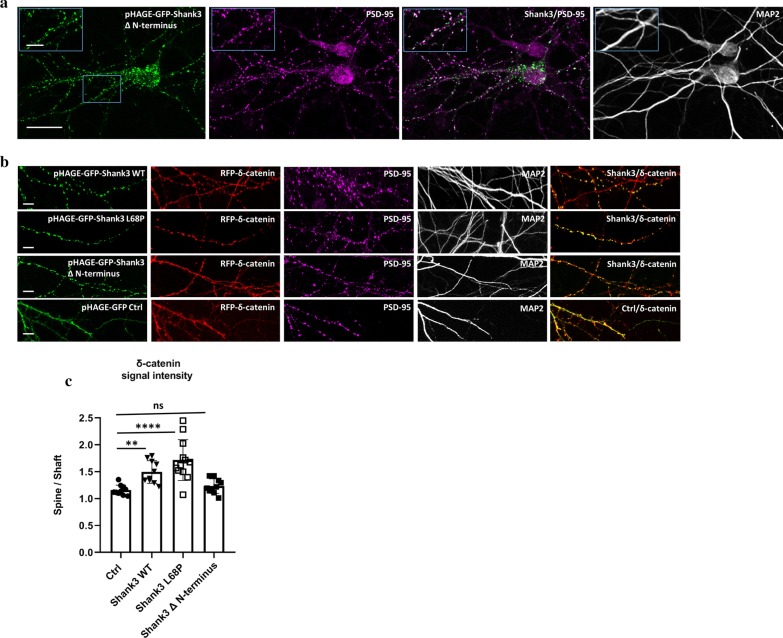
Fig. 7.
Contribution of the Shank3 N-terminus to the postsynaptic targeting of δ-catenin. a A new Shank3 construct lacking the N-terminal SPN-Ank domain was used for transfection of primary rat hippocampal neurons. The transfected neurons were fixed and stained for MAP2 and PSD-95. The green signal of Shank3 shows a high colocalization rate with PSD-95 indicating that the synaptic localization of Shank3 is not dependent on N-terminal domain (scale bar for overview images: 20 µm and for magnified images: 5 μm). b RFP-δ-catenin was coexpressed with either an empty pHAGE-GFP construct as a control or with different Shank3 (WT, L68P or ΔN-terminal) constructs. The neurons were fixed and stained for MAP2 as dendritic marker and PSD-95 as synaptic marker (scale bar 5 µm). c Quantitative analysis of δ-catenin signal intensity in 100 spines of 10 neurons obtained from two independent transfection, showed L68P variant of Shank3 increases the δ-catenin level in dendritic spines compared to Shank3 WT, whereas ΔN-terminal Shank3 is unable of changing localization of δ-catenin in neurons and shows a similar effect as pHAGE-GFP control construct. The results show that Shank3 N-terminus is responsible for recruiting δ-catenin to the postsynaptic sites (n = 10, one-way ANOVA with Dunnett’s Test, **p < 0.01, ****p < 0.0001, mean ± SD)