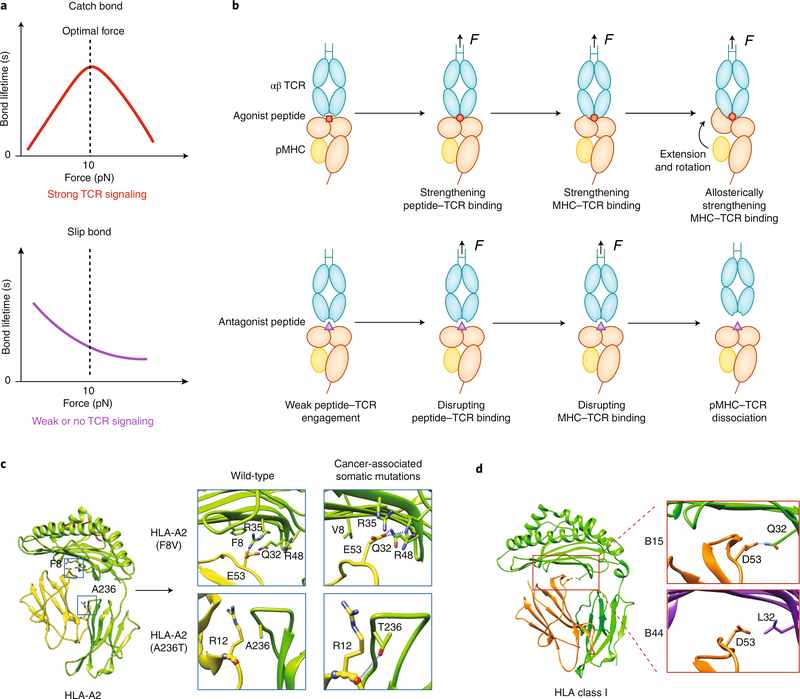
Fig. 3 |. Force-regulated pMHC conformations determine TCR–MHC dynamic bonds.
a, When a TCR forms a catch bond with an agonistic pMHC (top), increasing force prolongs the bond lifetime at low forces, but the bond changes to a slip bond at high forces. When a TCR forms a slip bond with antagonistic pMHC (bottom), increasing force monotonically shortens the bond lifetime. b, A TCR catch bond with agonistic pMHC is initiated by force-enhanced engagement between the TCR’s CDR3 loops and the peptide’s ‘hotspot’ residues, followed by a force-induced betterment of the complementarity of the MHC–TCR interface (top). Force also induces separation of β2m from the MHC heavy chain, which leads to MHC extension and rotation of the α1α2 domains toward the TCR; this further strengthens the TCR–MHC engagement allosterically. For a weak ligand, force is unable to induce the conformational changes noted above in the pMHC, which leads to slip bonds (bottom). c,d, Cancer-associated somatic mutations (c) or genotype variations (d) encoding mutant HLA molecules may also affect the susceptibility of MHC to conformational changes under force. Examples of the former are HLA-A2 F8V and A236, which form more hydrogen bonds between β2m and the MHC heavy chains (c). As an example of the latter, HLA-B15 has more hydrogen bonds between β2m and the MHC heavy chain than does HLA-B44 (d). Diagrams in Fig. 3c,d are modified from ref. 19.