To the Editor,
Severe eosinophilic asthma is characterized by increased blood eosinophil levels and recurrent exacerbations, and often associated with nasal polyposis. 1 Eosinophil cationic protein (ECP) and eosinophil‐derived neurotoxin (EDN), granule proteins released by eosinophils, are markers of eosinophil activation and have been identified as potential biomarkers of type 2 eosinophilic disease in patients with asthma. 2 , 3 , 4
The anti‐interleukin (IL)‐5 monoclonal antibody mepolizumab has been shown to reduce peripheral blood eosinophil counts (PBEC) and asthma exacerbations versus placebo in clinical studies. 5 , 6 , 7 Elevated PBEC and frequent exacerbations are key determinants for predicting patients with severe asthma who are most likely to respond to mepolizumab; 6 , 8 however, identifying additional biomarkers may enhance patient selection. This post hoc analysis of data from the Phase III MENSA study (GSK ID: 115588; NCT01691521) 7 investigated the relationship between baseline type 2 biomarkers and clinical outcomes in patients with severe asthma receiving mepolizumab.
MENSA was a randomized, double‐blind trial in patients aged ≥12 years with severe eosinophilic asthma. 7 Patients were randomized (1:1:1) to receive mepolizumab 75mg intravenously (IV) or 100 mg subcutaneously (SC), or placebo every 4 weeks for 32 weeks plus standard of care (see Appendix S1 for further details). Levels of the biomarkers EDN, ECP, chemokines (CCL‐13, CCL‐17, CCL‐22 and eotaxin‐1), periostin and IL‐13 were determined using serum samples taken at Weeks 0 (randomization) and 32 (exit).
Endpoints included the following: annualized rate of clinically significant exacerbations (defined as worsening of asthma requiring systemic corticosteroids for ≥3 days and/or hospitalization/emergency department visit); ratio to baseline of PBEC; change from baseline in prebronchodilator forced expiratory volume in 1 second (FEV1), asthma control questionnaire (ACQ)‐5 score, St George's Respiratory Questionnaire (SGRQ) total score (all at Week 32). Post hoc analysis assessments included baseline biomarker levels by PBEC subgroups (<150, 150‐<300, 300‐<500, ≥500 cells/μL) and presence of nasal polyposis at screening, correlation between biomarker levels and PBEC at baseline, and change from baseline in biomarker levels at Week 32. Additionally, the ratio of PBEC to baseline, changes from baseline in FEV1, ACQ‐5 and SGRQ scores, and annualized exacerbations (all at Week 32) were assessed in high (>median) and low (≤median) EDN (median = 57.6 μg/L) or ECP (median = 21.06 μg/L) subgroups at baseline (initial results determined which biomarkers were analysed further [EDN and ECP]). The annualized exacerbation rate was also assessed in high/low EDN and ECP subgroups further stratified by PBEC; baseline EDN level and baseline PBEC as predictors of response to mepolizumab treatment were also evaluated. Statistical analyses are described in Appendix S1. The MENSA trial was conducted in accordance with all applicable country‐specific regulatory requirements, and all patients provided written informed consent. Ethical approval was not required for this post hoc analysis.
In MENSA, 194 patients received mepolizumab 100 mg SC, 191 received mepolizumab 75 mg IV and 191 received placebo. At baseline, levels of most biomarkers increased with increasing PBEC, and the biomarkers EDN, ECP and IL‐13 showed a moderately positive correlation with PBEC (Table S1). Patients with nasal polyposis also had numerically higher baseline PBEC, EDN, ECP, CCL‐13, CCL‑17 and IL‐13 levels than those without nasal polyposis (data not shown). EDN was reduced by 70% from baseline to Week 32 with mepolizumab versus placebo, with a similar trend noted for ECP (58% reduction) (Figure S1A). In contrast, there was a trend for an increase in the levels of CCL‐13, CCL‐17, CCL‐22 and eotaxin‐1 between baseline and Week 32 with mepolizumab versus placebo (Figure S1B).
Owing to the positive correlation of EDN and ECP with PBEC, and their use as markers of eosinophil activation, we investigated clinical outcomes following mepolizumab treatment in patients with differing baseline EDN and ECP levels. Baseline PBEC was higher in patients with high versus low EDN or ECP levels at baseline and mepolizumab significantly reduced PBEC between baseline and Week 32 to a similar extent in both high and low EDN and ECP subgroups versus placebo. Mepolizumab‐induced improvements in other clinical outcomes were also numerically greater in high versus low baseline EDN or ECP subgroups (Table S2); as such, EDN and ECP may predict improvements in FEV1.
We also found mepolizumab reduced the exacerbation rate versus placebo in both baseline EDN subgroups, with a greater reduction in patients with high versus low EDN (Figure 1). To further investigate whether this effect could be explained in terms of confounding by baseline PBEC, subgroups were stratified by baseline PBEC. In patients with baseline PBEC ≥ 300 cells/µL, there was a larger reduction in exacerbations with mepolizumab in the high (75%) versus low (28%) baseline EDN subgroups. A similar trend was noted between the two < 300 cells/µL EDN subgroups. However, no difference was observed in the ECP subgroups. Furthermore, predicted rate ratio modelling of exacerbations demonstrated that a predictive model based on baseline PBEC was marginally better than that based on baseline EDN in terms of precision and model fit (Figure 2).
Figure 1.
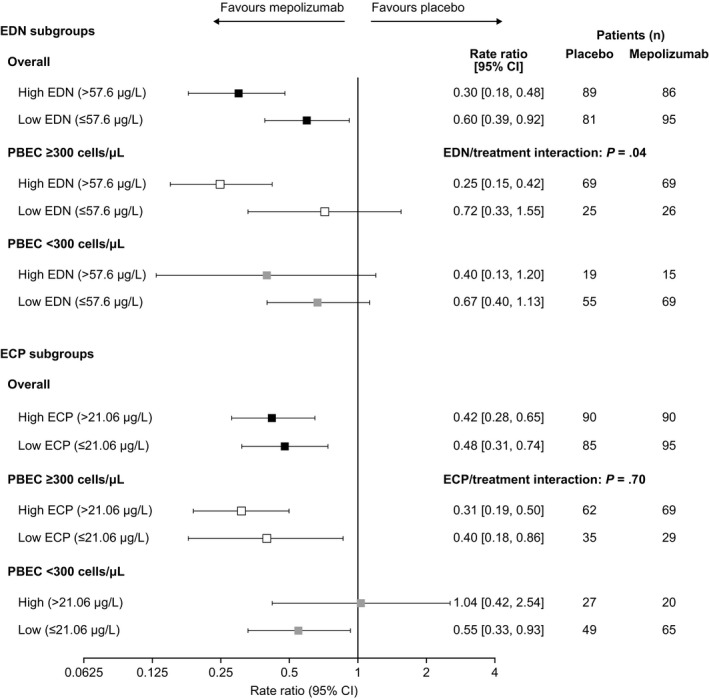
Ratio of the annual rate of clinically significant exacerbations with mepolizumab 100 mg SC versus placebo by baseline biomarker and PBEC subgroup. CI, confidence interval; ECP, eosinophil cationic protein; EDN, eosinophil‐derived neurotoxin; PBEC, peripheral blood eosinophil count; SC, subcutaneous
Figure 2.
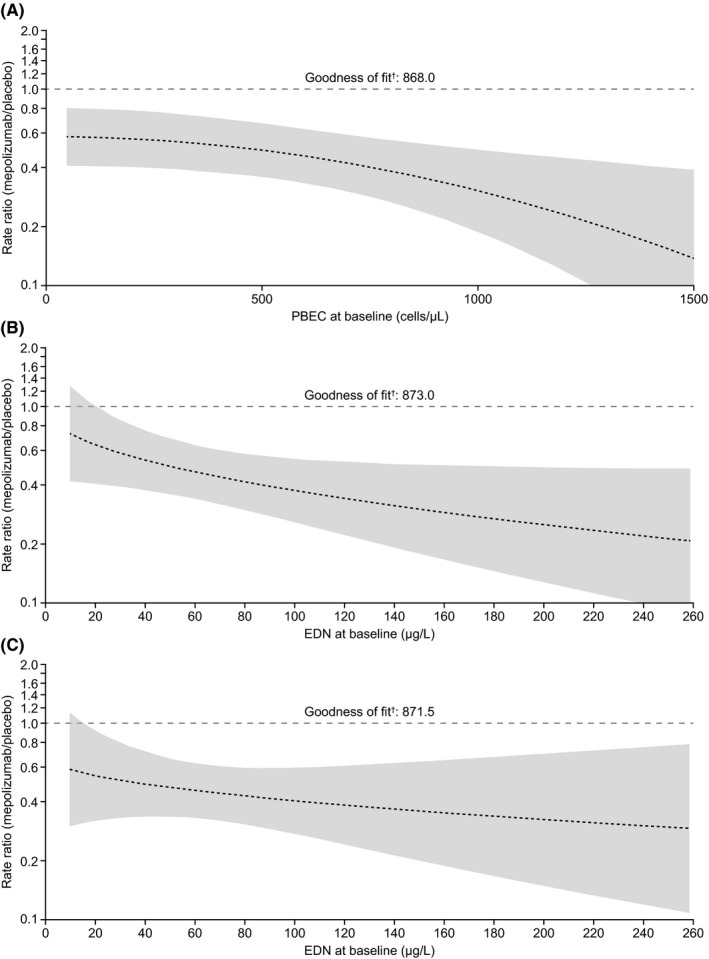
Predicted rate ratio (95% CI) of mepolizumab 100 mg SC versus placebo for clinically significant exacerbations per year versus (A) baseline PBEC, (B) baseline EDN concentration and (C) baseline EDN concentration adjusted for baseline PBEC and treatment interaction. †Akaike information criterion; lower values indicate a better model fit. Shading indicates 95% CI; wider bands indicate lower precision in predicting exacerbation rate. All analyses performed using a negative binomial regression model with covariates: treatment, maintenance corticosteroid use, exacerbation number in previous year. Additional covariates: (A) baseline PBEC (square transformation) including additional term for treatment interaction; (B) baseline EDN concentration (square‐root transformation) with term for treatment interaction; (C) baseline EDN concentration (square‐root transformation) with term for treatment interaction and baseline PBEC (log transformation) with treatment interaction. CI, confidence interval; EDN, eosinophil‐derived neurotoxin; PBEC, peripheral blood eosinophil count; SC, subcutaneous
Overall, we found baseline PBEC correlated with baseline EDN levels, as reported elsewhere,9 with a more pronounced reduction in the placebo‐adjusted annualized exacerbation rate with mepolizumab in patients with high versus low baseline EDN levels (trend not seen with ECP). Modelling analysis results demonstrated EDN levels and the combination of EDN levels, PBEC and treatment interaction did not show improved predictive power versus that of PBEC alone for treatment response to mepolizumab regarding exacerbation reduction. This result indicates that the predictive power of EDN is largely due to its correlation with baseline PBEC and highlights that PBEC remains an important and clinically relevant biomarker for identifying patients with severe asthma who are likely to respond to mepolizumab treatment.
In conclusion, our results demonstrate that in general, patients with higher versus lower EDN levels are likely to have improved responses to mepolizumab, although further research is needed on the role of EDN as a potential biomarker for treatment response to mepolizumab. However, baseline PBEC has greater precision as a predictive biomarker of treatment response to mepolizumab than EDN and is more widely assessed in clinical practice. Our data provide further evidence that PBEC is the most suitable biomarker identified to date for identifying patients likely to respond to mepolizumab, although the identification of additional biomarkers may further aid patient selection in the future.
CONFLICTS OF INTEREST
PH, SM, SB, SY and NK are employees of GlaxoSmithKline (GSK) and hold stocks/shares. FCA is a former employee of GSK and holds stocks/shares. SQ has served as a consultant to AstraZeneca, Novartis, Sanofi, Genentech, TEVA, ALK, Mundipharma and GSK; and has received lecture fees from Chiesi, Novartis, GSK, Leti, AstraZeneca and Mundipharma. AP reports grants, personal fees, nonfinancial support and other from Chiesi, AstraZeneca, Boehringer Ingelheim, Mundipharma and TEVA; personal fees and nonfinancial support from Menarini, Novartis and Zambon; and personal fees from Sanofi, all outside the submitted work. EI has served as a consultant to and received personal fees from AstraZeneca, Equillium, GSK, Novartis, 4D Pharma, Pneuma Respiratory, Regeneron Pharmaceuticals, Sanofi, Sienna Biopharmaceuticals and TEVA; reports nonfinancial support from TEVA, and other from Vorso Corp; and has received clinical research grants from AstraZeneca, Genentech, Novartis and Sanofi.
TRIAL REGISTRATION
Data from this post hoc analysis are from the MENSA trial, which is registered on ClinicalTrials.gov (GSK ID: MEA115588; NCT01691521).
Supporting information
Appendix S1
Fig S1
ACKNOWLEDGMENTS
This post hoc analysis and the parent study (GSK ID: MEA115588; NCT01691521) were funded by GSK. Editorial support (in the form of writing assistance, including development of the initial draft, assembling tables and figures, collating authors comments, grammatical editing and referencing) was provided by Roisin McCorkell, MSc, and Laura Gardner, PhD, of Fishawack Indicia Ltd, UK, and was funded by GSK.
DATA AVAILABILITY STATEMENT
Anonymized individual participant data from the study listed within this publication and its associated documents can be requested for further research from www.clinicalstudydatarequest.com.
REFERENCES
- 1. Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012;42(5):650‐658. [DOI] [PubMed] [Google Scholar]
- 2. Mogensen I, Alving K, Bjerg A, et al. Simultaneously elevated exhaled nitric oxide and serum‐eosinophil cationic protein relate to recent asthma events in asthmatics in a cross‐sectional population‐based study. Clin Exp Allergy. 2016;46(12):1540‐1548. [DOI] [PubMed] [Google Scholar]
- 3. Gon Y, Ito R, Hattori T, et al. Serum eosinophil‐derived neurotoxin: correlation with persistent airflow limitation in adults with house‐dust mite allergic asthma. Allergy Asthma Proc. 2015;36(6):e113‐120. [DOI] [PubMed] [Google Scholar]
- 4. Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289(25):17406‐17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. GlaxoSmithKline . NUCALA US prescribing information. https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Nucala/pdf/NUCALA‐PI‐PIL‐IFU‐COMBINED.PDF. Accessed January 9, 2020. [Google Scholar]
- 6. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add‐on therapy on health‐related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double‐blind, placebo‐controlled, parallel‐group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390‐400. [DOI] [PubMed] [Google Scholar]
- 7. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198‐1207. [DOI] [PubMed] [Google Scholar]
- 8. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double‐blind, placebo‐controlled trial. Lancet. 2012;380(9842):651‐659. [DOI] [PubMed] [Google Scholar]
- 9. Pham TH, Damera G, Newbold P, Ranade K. Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respir Med. 2016;111:21‐29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Fig S1
Data Availability Statement
Anonymized individual participant data from the study listed within this publication and its associated documents can be requested for further research from www.clinicalstudydatarequest.com.


