Figure 2.
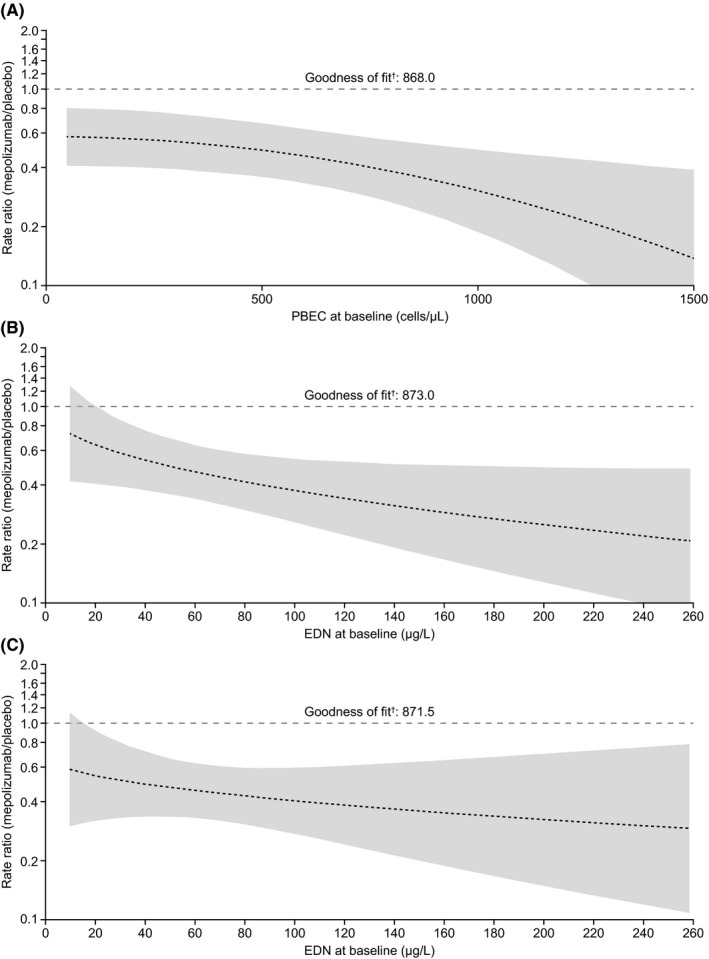
Predicted rate ratio (95% CI) of mepolizumab 100 mg SC versus placebo for clinically significant exacerbations per year versus (A) baseline PBEC, (B) baseline EDN concentration and (C) baseline EDN concentration adjusted for baseline PBEC and treatment interaction. †Akaike information criterion; lower values indicate a better model fit. Shading indicates 95% CI; wider bands indicate lower precision in predicting exacerbation rate. All analyses performed using a negative binomial regression model with covariates: treatment, maintenance corticosteroid use, exacerbation number in previous year. Additional covariates: (A) baseline PBEC (square transformation) including additional term for treatment interaction; (B) baseline EDN concentration (square‐root transformation) with term for treatment interaction; (C) baseline EDN concentration (square‐root transformation) with term for treatment interaction and baseline PBEC (log transformation) with treatment interaction. CI, confidence interval; EDN, eosinophil‐derived neurotoxin; PBEC, peripheral blood eosinophil count; SC, subcutaneous
