Figure 1.
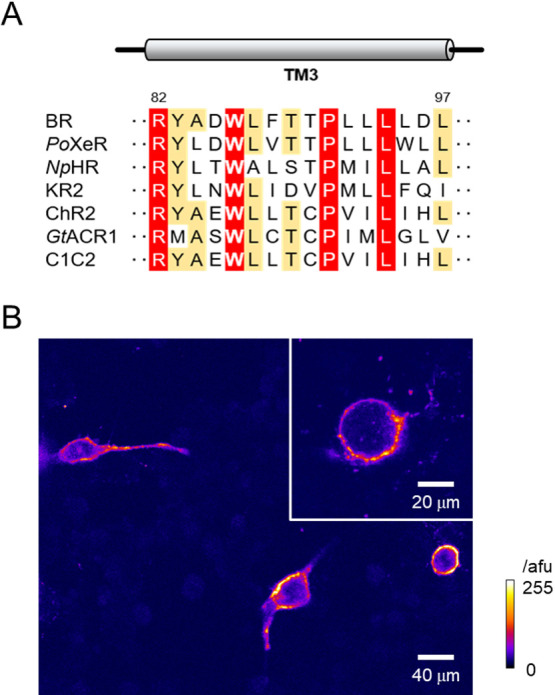
A highly conserved helix-3 tryptophan and its mutation in C1C2/ChRWR. (A) Amino acid sequence alignment of the third transmembrane domain (TM3) from microbial rhodopsins with various ion transport activities. The highly conserved and moderately conserved residues are highlighted in red and orange, respectively. The residue numbers of BR are shown on top of the alignment. (B) A typical confocal image (a single slice) of transfected C1C2/ChRWR (hWR) with W163F mutation (hWR-W163F) in ND7/23 cells (13 days after transfection). Inset, a zoom image of another sample. Note the membrane-delimited expression of Venus fluorescence the intensity of which was scaled by the arbitrary fluorescent unit (afu) and expressed in pseudocolor ratings.
