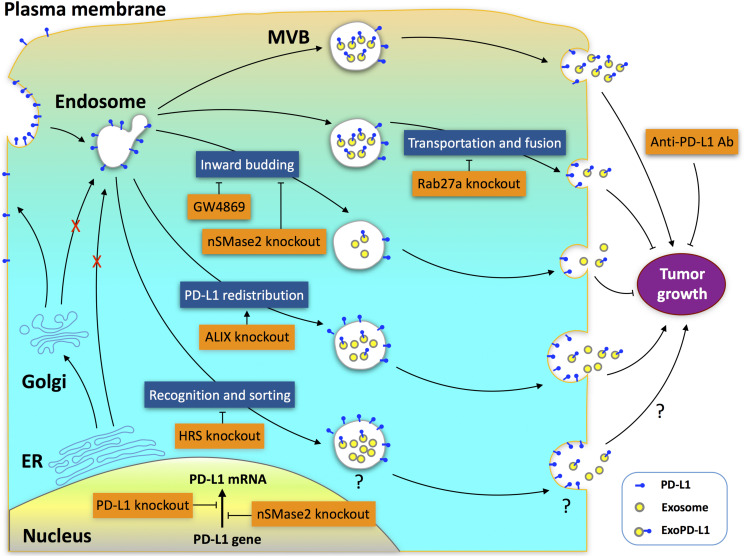
FIGURE 2.
Potential targets for antitumor therapy in ExoPD-L1 biogenesis pathways. Multiple molecules, including Rab27a, nSMase2, ALIX, and HRS, participate in the complex processes of ExoPD-L1 biogenesis, which originates from the cell surface rather than from the ER or Golgi apparatus. Deletion of Rab27a decreases ExoPD-L1, but does not alter cell-surface PD-L1 levels. Deletion of nSMase2 reduces the levels of both cellular PD-L1 and ExoPD-L1 protein. Rab27a deletion causes a greater inhibition in exosome production compared with nSMase2 deletion, while nSMase2 deletion leads to a greater inhibition of ExoPD-L1 production compared with Rab27a deletion. GW4869, an inhibitor of nSMase2, inhibits ExoPD-L1 generation, but does not increase cellular PD-L1 levels. Knockdown of ALIX, which redistributes PD-L1 between the cell-surface and exosomes, results in a reduction of ExoPD-L1 production but an increase in cell-surface PD-L1. Blockade of Rab27a or nSMase2 results in suppression, whereas ALIX knockdown promotes tumor growth. Knockdown of HRS, an ESCRT-0 subunit, confers a decrease in ExoPD-L1 levels but an increase in cellular PD-L1 levels. The effects of HRS knockdown on the cell-surface PD-L1 levels and tumor growth remain unknown.