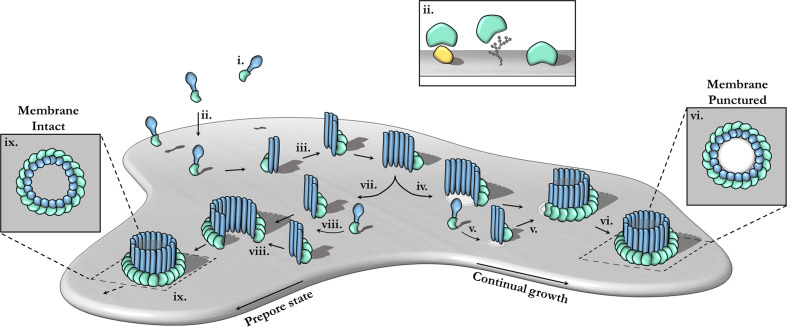
Figure 3.
The canonical pathway of pore formation. A generic pore forming protein is shown, with a green ancillary (or receptor binding) domain and blue pore forming domain. Freely diffusing monomers (i) bind to the target bilayer (gray) (ii) via target recognition domains (green) that are ancillary to the pore forming machinery (blue). The target receptor can be proteins (yellow), glycans or lipids (ii; inset). Membrane-bound monomers undergo two-dimensional diffusion, colliding and eventually oligomerizing (iii). Maturation via the prepore-to-pore conformational change may occur at different stages. For example, incomplete oligomers may transition into arc-pores (iv). Other smaller arcs or monomers may also be recruited to a growing arc pore (v). Ultimately complete pores are formed upon the closure of the oligomeric ring (vi). In this context pore growth can occur in a continuous mechanism. Completed pores define large aqueous channels, capable of facilitating the passive diffusion of additional effector molecules (not shown) via the membrane channel (vi; inset). Alternatively, arc prepores may continue to grow without inserting into the membrane (vii) by recruiting additional monomers or other smaller arcs (viii). These can ultimately form complete prepores that have yet to punch into the lipid bilayer (ix). Fully formed prepores are most commonly observed for CDCs (ix). The prepore-to-pore transition is triggered resulting in a conformational change of the MACPF core machinery that unfurls into a giant β-barrel (ix goes to vi). These inserted pores possess an amphipathic region that is fully inserted into the lipid membrane (not shown). Insets (ix, vi) show top-down views.