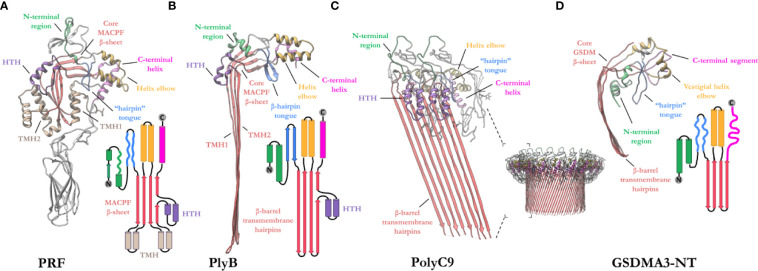
Figure 4.
Exemplar structures of MACPF pore forming proteins in the monomeric and pore states. (A) Crystal structure of lymphocyte PRF in the monomeric state [PDB: 3NSJ] (43). The ancillary domain is colored gray and omitted from the topology diagram for clarity. (B) The cryoEM pore structure of the fungal MACPF protein, pleurotolysin (PlyB)[PDB: 4V2T] (13). Pleurotolysin is a homolog of PRF found in oyster mushroom. The β-trefoil domain of PlyB is not shown for clarity. (C) A dimer of the polyC9 cryoEM reconstruction (bottom right) is shown to illustrate the intra-subunit contacts at the MACPF interface [PDB: 6DLW] (22, 23). (D) The cryoEM pore structure of the intracellular GSDMA3-NT shows structural and topological similarity to the MACPF domain [PDB: 6CB8] (103). HTH, helix-turn-helix (purple); TMH, transmembrane β-hairpin (pale brown). Topology diagrams are colored consistently with the PDB coordinates.