Figure 3.
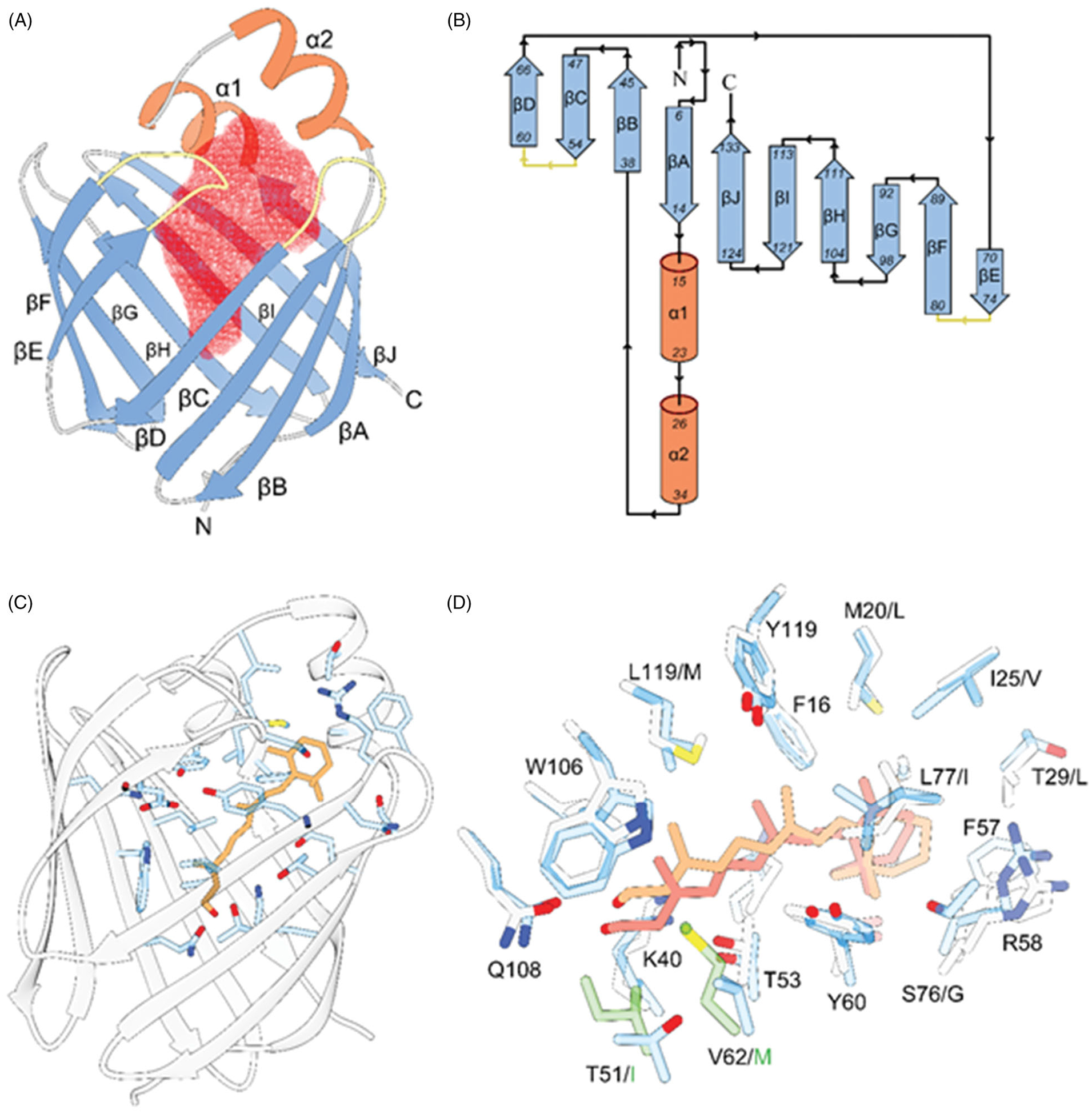
The overall structural motive and the mode of all-trans-retinol interaction with RBPs. A, ribbon diagram of human RBP2 (PDB #6BTH). The red mesh represents the ligand-binding cavity inside the β-barrel composed of ten antiparallel β-strands. The entry portal region is defined by two α-helices (shown in orange) and loops connecting and β-strands C-D and E-F (colored yellow). B, topology diagrams for the RBPs. The color scheme is identical as in panel A. C, position of the retinoid moiety within the binding pocket of RBP2 (PDB #4QZT). The side chains of amino acids in the vicinity of all-trans-retinol (orange) are highlighted in blue. D, overlay of the binding sites structures and ligand orientation found in human RBP1 (PDB #5HBS) and human RBP2 (PDB #4QZT). The side chains of RBP1 and RBP2 are colored gray and blue, respectively. The position of all-trans-retinol seen in RBP1 is shown in light orange, whereas the retinoid moiety found in RBP2 is colored in pink. The side chains shown in green correspond to the substitutions of T51/I and V62/M in RBP1 that contribute to the different in the position of the ligand.
