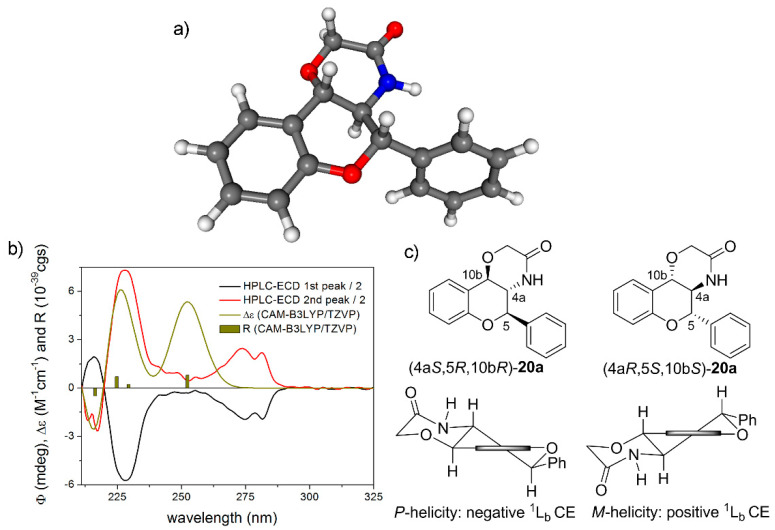
Figure 7.
(a) Single low-energy CAM-B3LYP/TZVP PCM/CHCl3 conformer of (4aR,5S,10bS)-20a containing a flavan chromophore with M-helicity. (b) HPLC-ECD spectra of the first (black line) and the second-eluting (red line) enantiomers of 20a compared with the CAM-B3LYP/TZVP PCM/CHCl3//CAM-B3LYP/TZVP PCM/CHCl3 spectrum of (4aR,5S,10bS)-20a (olive line). The bars represent rotational strength values for the single low-energy solution conformer. (c) Structure and helicity of the separated enantiomers of rac-20a. Horizontal thick line represents the plane of the condensed benzene ring.