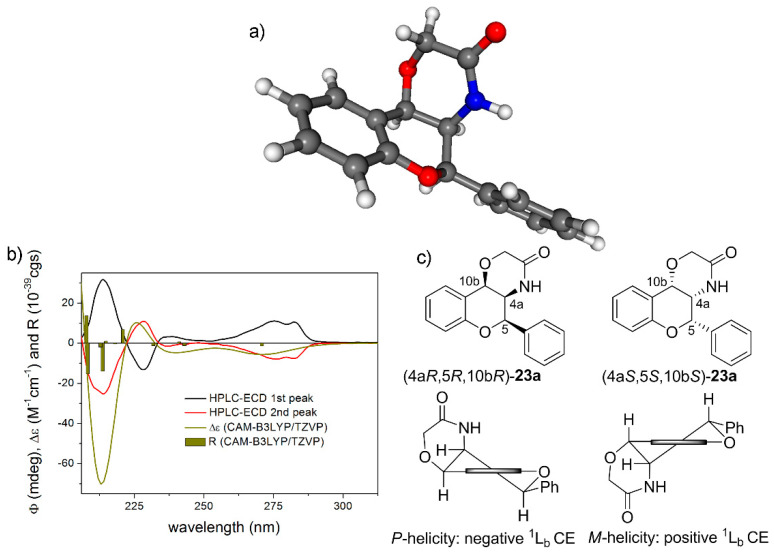
Figure 8.
(a) Single low-energy CAM-B3LYP/TZVP PCM/CHCl3 conformer of (4aR,5R,10bR)-23a containing a flavan chromophore with P-helicity. (b) HPLC-ECD spectra of the first- (black line) and the second-eluting (red line) enantiomers of rac-23a compared with the CAM-B3LYP/TZVP PCM/CHCl3//CAM-B3LYP/TZVP PCM/CHCl3 spectrum of (4aR,5R,10bR)-23a (olive line). The bars represent rotational strength values for the single low-energy solution conformer. (c) Structure and helicity of the (4aR,5R,10bR)-23a and (4aS,5S,10bS)-23a. Horizontal thick line represents the plane of the condensed benzene ring.