Table 1.
Steroid intermediates detected during cholesterol bioconversion by N. simplex VKM Ac-2033D.
| Number | Name | Molecular Weight | Chemical Structure | Number | Name | Molecular Weight | Chemical Structure |
|---|---|---|---|---|---|---|---|
| V | Cholest-4-en-3-one | 384 |
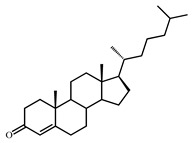
|
VI | Cholesta-1,4-dien-3-one | 382 |
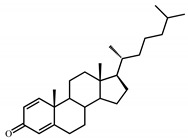
|
| VII | 26-Hydroxy-cholest-4-en-3-one | 400 |
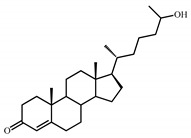
|
VIII | 3-Oxo-cholest-4-en-26-oic acid | 414 |
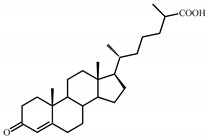
|
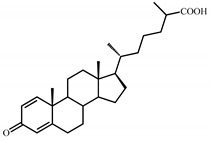
| IX | 3-Oxo-cholesta-1,4-dien-26-oic acid | 412 |
|
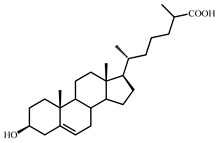
X | 3β-Hydroxy-cholest-5-en-26-oic acid | 416 |
|
| XI | Androst-4-en-17β-ol-3-one, Testosterone |
288 |
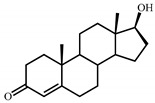
|
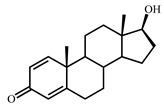
XII | Androsta-1,4-dien-17β-ol-3-one, 1-Dehydrotestosterone | 286 |
|
| XIII | Androst-4-ene-9α,17β-diol-3-one, 9α-Hydroxy-testosterone(preliminarily determined) | 304 |
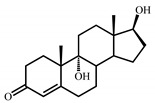
|
