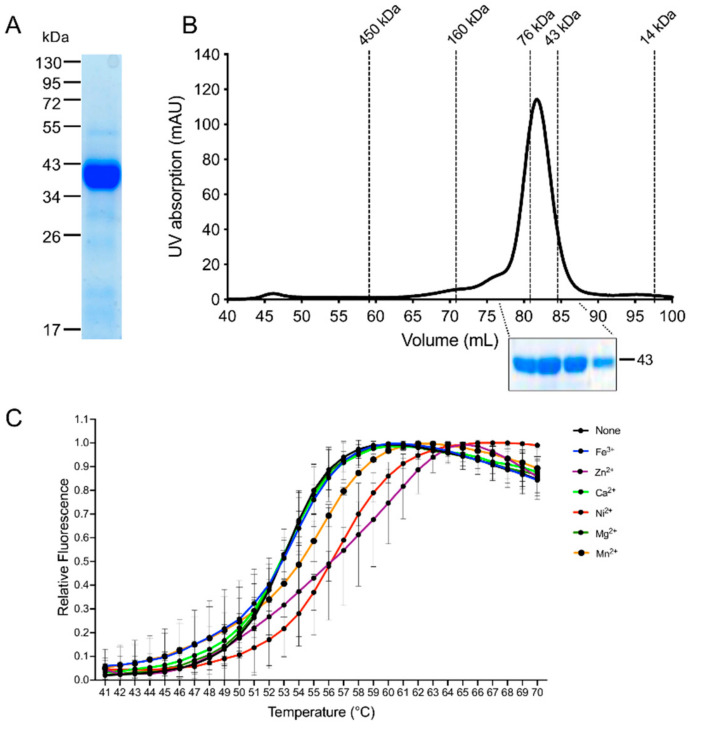
Figure 3.
Recombinant expression, investigation of the oligomeric state and putative metal cofactor binding of EhADH3Bb. (A). ehadh3bb was recombinantly expressed in E. coli as a GST-fusion protein and purified using affinity chromatography. After purification and cleavage of the GST tag, the recombinant ADH3Bb (recADH3Bb) can be displayed as a 43 kDa protein band after SDS-PAGE and Coomassie staining. (B). Size exclusion chromatography of recADH3Bb was performed on a HiLoad 16/60 Superdex prep grade column in a buffer containing 50 mM Tris, 150 mM NaCl, 10 mM EDTA, 1 mM DTT, 10% glycine, pH 8.0. Elution peaks of standard proteins are indicated by dotted lines in the elution profile of the target protein (black solid line). An elution peak of the target protein at 82 mL and subsequent detection of the protein in the respective elution fractions by SDS-PAGE indicates that recADH3Bb is present as a dimer in solution. (C). Thermal stabilization assay for the determination of the putative metal cofactor of recEhADH3Bb by addition of different metal ions at a concentration of 2 mM. The melting curve of recEhADH3Bb without addition of metal ions was used as a control for the assay. The relative fluorescence is displayed as a function of temperature.