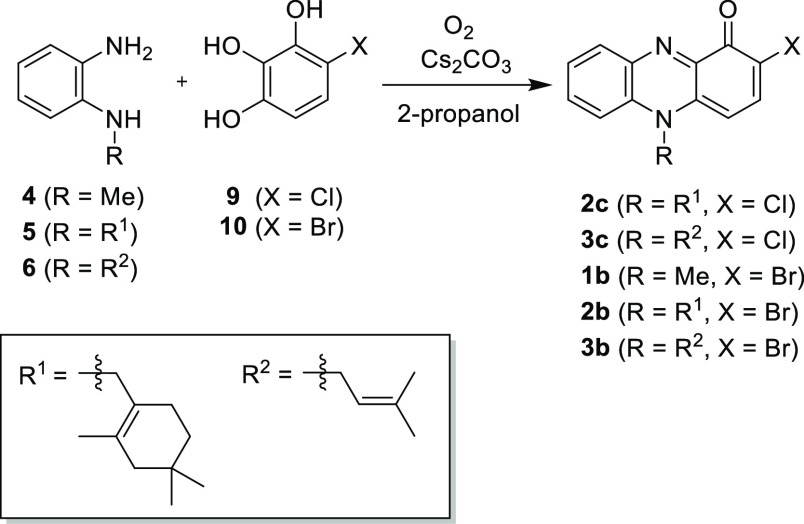
Table 2. Oxidative Condensation of 4-Halo-1,2,3-benzenetriols with N-Alkylbenzene-1,2-diaminea.
| entry | 1,2-diamine | 4-halo-1,2,3-benzenetriols | product | yield (%)b |
|---|---|---|---|---|
| 1c | 5 | 9 | 2c | 20 |
| 2d | 6 | 9 | 3c | 21 |
| 3d | 4 | 10 | 1b | 18 |
| 4c | 5 | 10 | 2b | 10 |
| 5d | 6 | 10 | 3b | 15 |
Unless otherwise noted, reactions were performed at rt, with O2 bubbling or under an oxygen atmosphere, using N-alkylbenzene-1,2-diamine (30.0 mg) and 4-halo-1,2,3-benzenetriols (1.0 equiv) in the presence of Cs2CO3 (1.0 equiv).
Isolated yield.
The reaction was performed with oxygen bubbling.
The reaction was performed under an oxygen atmosphere.