Figure 6.
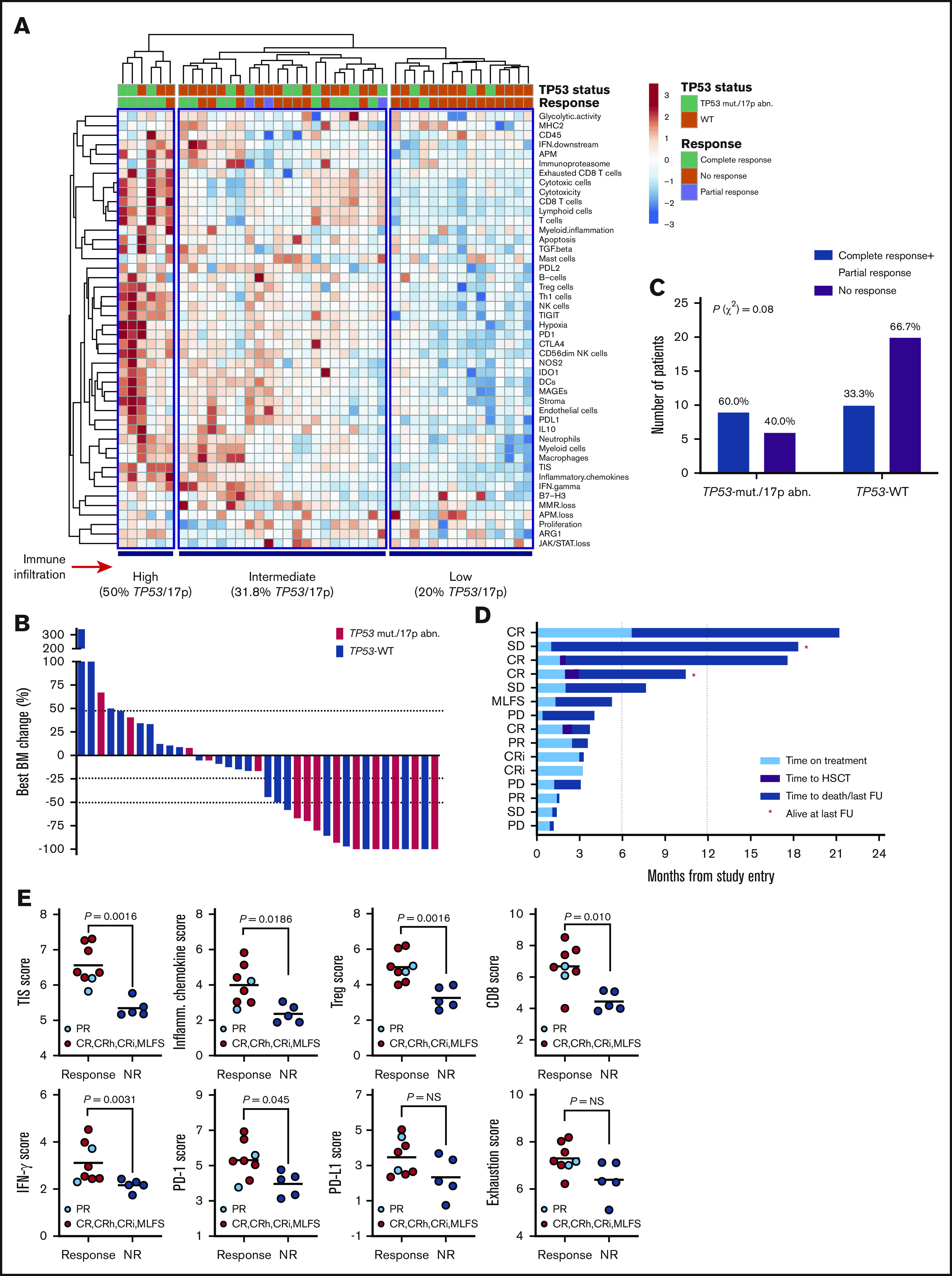
Immune landscape and immunotherapy response in patients with relapsed/refractory AML with and without TP53 mutations and/or 17p abnormalities. (A) Heat-map of immune cell type-specific scores and biological activity scores in patients with relapsed/refractory AML treated with flotetuzumab immunotherapy. ClustVis, an online tool for clustering of multivariate data, was used for data analysis and visualization.47 (B) Waterfall plot depicting changes in BM blasts after cycle 1 of flotetuzumab in patients with TP53 mutations and/or 17p deletion (n = 14; a BM sample was not available in 1 patient who progressed on treatment. (C) Response to flotetuzumab in relation to TP53 abnormalities (χ2 test). (D) Swimmer plot showing time on treatment and time to death and/or censoring in relation to clinical response in the 15 patients with TP53 mutations and/or 17p deletion with genomic loss of TP53. Response criteria are described in "Materials and methods." (E) TIS, inflammatory chemokine, Treg cell, CD8, IFN-γ, PD1, PD-L1, and exhaustion mRNA scores in baseline BM samples from patients with TP53 mutations and/or 17p deletion. Complete responses were defined as either CR, CR with partial hematological recovery, CRi, or MLFS. Data were compared using the Mann-Whitney U test for unpaired determinations. CR, complete remission; CRh, complete remission with partial hematopoietic recovery; FU, follow-up; OB, other benefit (>30% decrease of BM blasts relative to baseline); NR, no response; PD, progressive disease; SD, stable disease.
