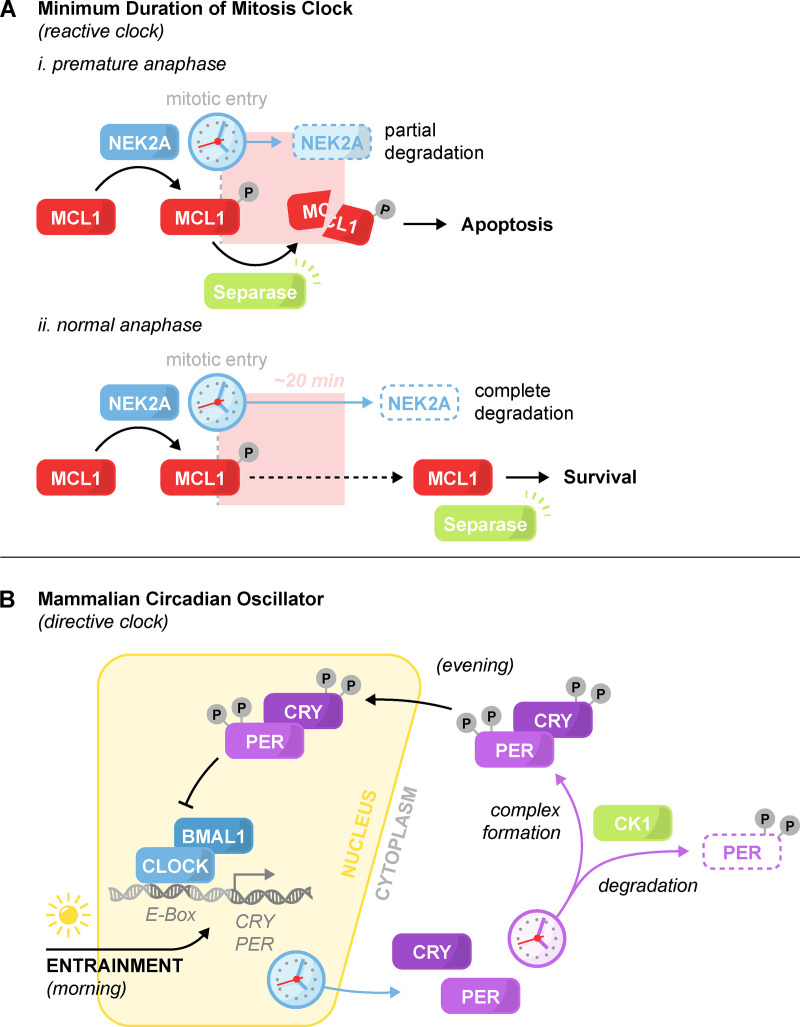
Figure 3.
Minimum duration of mitosis clock and the mammalian circadian oscillator. The architecture of a reactive and directive clock. (A) Minimum duration of mitosis clock. The timekeeper NEK2A degrades at the onset of mitosis. Phosphorylation of the antiapoptotic protein MCL1 by NEK2A sensitizes MCL1 to Separase cleavage. (i) In the event of premature anaphase onset, Separase activation triggers apoptosis. (ii) In normal mitosis, degradation of NEK2A relieves MCL1 phosphorylation and the sensitivity to Separase activation, thereby enabling mitotic progression. (B) Simplified schematic of the core mammalian circadian oscillator. At dawn, the transcription factor CLOCK:BMAL1 initiates the transcription and accumulation of its negative regulators PER and CRY. In the evening, nuclear translocation of PER and CRY lead to CLOCK:BMAL1 repression and PER and CRY depletion. Depletion of PER and CRY de-represses CLOCK:BMAL1 and restarts the cycle. Dozens of gradual phosphorylations by CK1 promote the degradation, complexing, and nuclear translocation of PER and CRY. Light-induced transcription of PER and CRY is the primary mechanism for clock entrainment. In addition to what is shown, other feedback loops and kinases also help maintain the clock’s pace.