Figure 3.
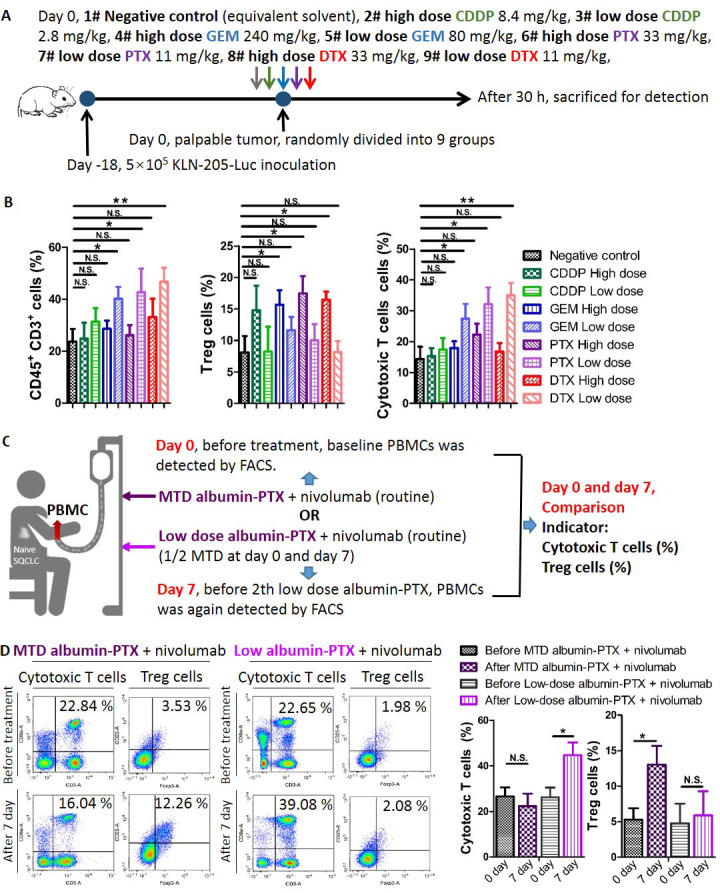
High-dose SQCLC monochemotherapy induce greater immunosuppression compared with low-dose regimens in vivo. (A) Chemotherapy treatment scheme. Syngeneic mouse models were established as mentioned above. When tumors became palpable, mice were randomly divided into nine groups receiving an intraperitoneal injection of low-dose CDDP 2.8 mg/kg, high-dose CDDP 8.4 mg/kg, low-dose GEM 60 mg/kg, high-dose GEM 240 mg/kg, low-dose PTX 11 mg/kg, high-dose PTX 33 mg/kg, low-dose DTX 11 mg/kg, high-dose DTX 33 mg/kg, or vehicle. (B) After 30 hours, tumors were harvested for FACS analysis. (C, D) Patients with reatment-naïve SQCLC received either MTD albumin-PTX (at day 0)+nivolumab (routine administration) or low-dose albumin-PTX (1/2 MTD at day 0 and day 7)+nivolumab (routine administration). Before treatment (day 0), baseline PBMCs were evaluated by FACS. At day 7 (before second low-dose albumin-PTX), PBMCs were again assessed by FACS. Cell fractions (cytotoxic T cells and Treg cells) before and after treatment were then compared. Data are presented as mean±SD. N.S., no significance, *p<0.05; **p<0.01; ***p<0.001. CDDP, cisplatin; DTX, docetaxel; FACS, fluorescence-activated cell sorting; GEM, gemcitabine; Luc, luciferase; MTD, maximum tolerated dose; PBMC, peripheral blood mononuclear cell; PTX, paclitaxel; SQCLC, squamous cell lung carcinoma; Treg, regulatory T cell.
