Figure 1: Mutant calreticulin secreted by cancer cells inhibits their phagocytosis by dendritic cells.
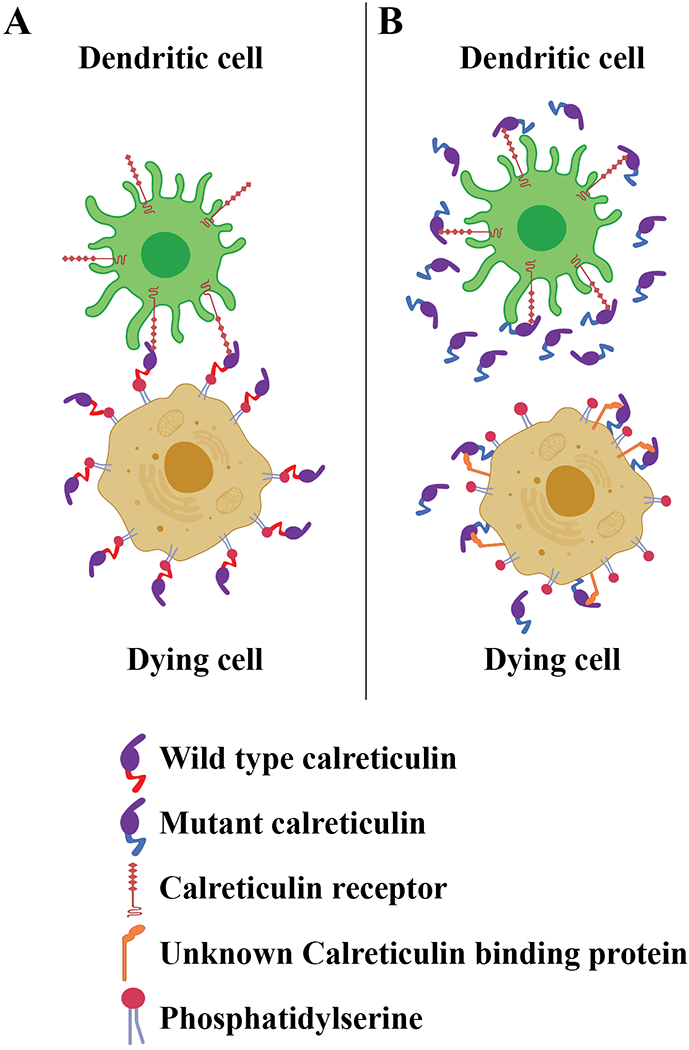
A) Dying cancer cells express cell-surface calreticulin under immunogenic cell death conditions, such as those induced by certain types of drug treatments (Galluzzi et al., 2015). The acidic C-terminal domain of wild type calreticulin is known to engage surface phosphatidylserine of apoptotic cells in a calcium-dependent manner, which is one mechanism of calreticulin anchoring to the cell surface (Wijeyesakere et al., 2016). Cell-surface calreticulin binds phagocyte receptors such as the lipoprotein-related receptor I (LRP-1) (Gardai et al 2005), to enhance phagocytic uptake and induction of protective anti-tumor T cell responses (Galluzzi et al., 2015).
B) Cancer cells express somatic mutants of calreticulin with basic C-terminal domains and lacking a KDEL sequence (Klampfl et al., 2013; Nangalia et al., 2013), and such mutants are known to be secreted (reviewed in How et al., 2019). Secreted forms of calreticulin are shown by Liu et al. to inhibit the phagocytosis of dying cancer cells by dendritic cells, presumably by saturating the calreticulin binding sites on the surface of dendritic cells. This results in reduced T cell-mediated anti-tumor immunity (Liu et al., 2020). The mode of calreticulin binding to the surface of dying cancer cells is also predicted to be altered by mutations of the C-terminal acidic domain of calreticulin to basic sequences, which need further assessment.
