Abstract
Purpose of Review
Colorectal cancer (CRC) is the second leading cause of cancer-associated deaths in the United States, with most metastatic cases subsequently turning refractory to standard chemotherapy. One of the promising current interventions is immunotherapy that relies on harnessing the body’s immune mechanisms to kill the cancer cells. The aim of this review is to highlight the implications of single versus combination immunotherapy and identify the molecular features and mutations that enhance or deter responsiveness.
Recent Findings
Based on current findings, responsiveness is associated with deficiency of mismatch repair (dMMR) genes or presence of microsatellite instability (MSI-high), with high immunoscore and tumor-mutational burden contributing to better efficacy while BRAF mutation conferring no significant effect. Combination immunotherapy demonstrates better efficacy in treating MSI-high CRC compared to single agent immunotherapy or chemotherapy.
Summary
Given improved responsiveness and overall survival, there is potential for immunotherapy to change the standard of care for metastatic CRC. Furthermore, stratifying the patients by their molecular features and mutation status is critical for establishing care.
Keywords: Colorectal Cancer, Immunotherapy, Microsatellite Instability, Mismatch-repair, Nivolumab, Pembrolizumab
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer-associated deaths worldwide, and the second major cause of cancer-associated deaths in the United States[1]. It is commonly known as a “silent killer” as the symptoms often do not appear until a late stage. Approximately 20–30% of CRC patients are diagnosed at an advanced stage, with those diagnosed earlier showing a high incidence of disease relapse. Treatment modalities are based on tumor-nodes-metastasis (TNM) staging system provided by American Joint Committee on Cancer/Union Internationale Contre le Cancer (AJCC/UICC), which relies on tumor characteristic, including the extent of the primary tumor (T), the involvement of regional lymph nodes(N) and the presence of metastases (M)[2]. However, the reliability of the TNM system in predicting CRC prognosis is debatable. This discrepancy has led to a substantial under- and over-treatment of CRC [3]. Current first-line treatment for CRC includes standard chemotherapy (fluorouracil, leucovorin, oxaliplatin, and irinotecan) plus bevacizumab (BV)) with surgery and/or radiation. Anti-EGFR therapy is also an important component of standard combination chemotherapy regimen specifically for left-sided CRC (distal transverse, descending, and sigmoid colon and rectum) and those lacking KRAS and NRAS mutation. Even with an arsenal of therapies, the 5-year survival rate for patients in stage I CRC is 90%, which drops abruptly to 14% for stage IV metastatic CRC[4], suggesting that with advance stage, CRC becomes progressively refractory to standard treatment, potentially owing to increased heterogeneity acquired with time. Therefore, there is an increasing need to improve disease stratification, identify biomarkers, and find treatment alternatives for late-stage disease.
Molecular Features of CRC
In clinical oncology, tumor-specific molecular features are increasingly being evaluated to make treatment decisions. Molecular testing involves analyzing both sporadic and hereditary mutations that can assist in predicting disease prognosis and determine the choice of treatment [5]. Some of the molecular features commonly seen in CRC include KRAS and BRAF gene mutations and microsatellite instability (MSI). BRAF-V600E mutation has a poor prognosis and constitutes ~10% of all metastatic cases. In general, CRC can be divided into four major consensus molecular subtypes (CMS): CMS1, CMS2, CMS3 and CMS4[6]. CMS1 constitutes 14% of all CRC cases and harbors high mutation-load, displays immune-infiltration, and is MSI-like. This subtype is also enriched for tumors hypermethylated at CpG islands (e.g. hypermethylated MLH1) as well as BRAF mutations (discussed later). CMS2, considered canonical subtype, makes 37% of CRC cases, is MSI-stable, and shows activated Wnt and Myc signaling. Similarly, CMS3, considered a metabolic subtype, constitutes another 13% of CRC cases and presents with frequent KRAS mutations. Finally, CMS4, considered a mesenchymal subtype, constitutes nearly 23% of all CRC cases and shows elevated activation of TGF-β pathways[7]. Further characterization of the CMS4 subtype has revealed a high density of fibroblast within the tumor microenvironment, which has been speculated to promote tumor microenvironment, tumor-associated inflammation and angiogenesis via secretion of chemokines and cytokines[8], all features associated with poor prognosis. Overall, these molecular features suggest that CRC subtypes can be largely divided based on their MSI status (presence or absence of mismatch repair (MMR)[9] genes including MLH1, MSH2, MSH6 and PMS2 [10]), mutation status of BRAF, KRAS and prolonged activation of proto-oncogenes such as wnt and myc.
Approximately 65% of CRCs are sporadic, with less than 5% CRCs with a genetic predisposition[11]. Of the sporadic cases, the majority (85%) of CRC exhibit chromosomal instability (CIN), copy number variations, rearrangements, loss of heterozygosity (LOH), among others[12]. The remaining (15%) exhibit MSI [13]. Hereditary CRC is less common and has two types: 1) Familial adenomatous polyposis (FAP) that results from mutation in adenomatous polyposis (APC) gene and 2) Non-polyposis Colorectal Cancer (NPCC, or Lynch syndrome), which results from microsatellite instability. Both sporadic and inherited forms of CRC display some degree of MSI.
Immunoscore (Immune-infiltration)_
One of the key features of dMMR and MSI-high CRCs (CMS1) is the presence of high-mutation load within the tumor, which is further characterized by infiltration of tumor-associated lymphocytes (TILs), including CD8+ T cells, IFN-γ producing activated Th1 cells and CD45 RO+ T memory cells [14]. This finding suggests that in this CRC subtype, the tumor microenvironment (TME) evolves to promote high mutation load and immune infiltration. Since TNM staging gives no insight into the immune status of cancer, adaptive immune cell infiltration status, rather than TNM staging, has been shown to have better prognostic value. An effort to translate immune-status into a prognostic marker was made by introducing “immunoscore”, which is based on the quantification of lymphocyte populations, in particular CD3 and CD8 positive T cells, both at the tumor center and at the invasive margin[15, 2]. The scoring system ranges from low immune cell densities (immunoscore 0) to high immune densities with a score of 4. According to early tumor analyses in CRC cases, a high immunoscore was found in 45% of MSI-high CRCs and 21% of MSS tumors[16]. This finding suggests that an immune-based scoring system provides a surrogate measure of immunogenicity that may have high utility in treating immune-infiltrated tumors, designated as “hot tumors.”
MSI-high constitutes 15% of all stage II/stage III CRC cases, and 4–5% of stage IV CRC cases[17]. Although MSI-high CRCs remain a small percentage of CRC cases, their refractoriness to standard chemotherapy [18] has garnered a lot of attention. Although refractory to standard chemotherapy, their high mutation load confers them marked immunogenicity, and therefore, renders them particularly susceptible to immunotherapy. Below we discuss some of the current immunotherapies that have been approved or are under evaluation for dMMR/MSI high CRC cases. We also shed some light on the potential of immunotherapy for pMMR (MMR proficient)/MSS CRC cases.
Immunotherapy in CRC
Immunotherapy relies on harnessing the body’s immune system to kill cancer cells[19]. In healthy individuals, transformed cells that arise naturally due to defects in apoptosis or cell cycle checkpoints are targeted for killing by the immune system. Due to their altered transcription and translational machinery, cancer cells give rise to novel antigens (neo-antigens) that are seen as “foreign” by the immune system and, therefore, are targeted for cytotoxic killing by CD8+ T cells (Figure 1A). For the effective killing of cancer cells, cytotoxic T cells need prolonged co-stimulation and simultaneous blockade or weakening of checkpoints that inhibit T-cell activation. Some of the well-known checkpoints involved in T-cell inhibition include PD1 and CTLA-4 receptors that are expressed by T-cells as a mechanism to regulate prolonged activation. However, the expression of these receptors within a tumor microenvironment renders T-cells ineffective. To oppose T-cell mediated killing and transmit inhibitory signal, tumor cells express cognate ligands PDL-1, B7.1 and B7.2 that bind PD1 and CTLA-4, respectively, and therefore dampen overall immune response (Figure 1A–B). Tumors that are characterized by infiltrating lymphocytes expressing these checkpoints have, therefore, been targeted with several checkpoint blockade (anti-PD1, anti-PDL1, anti-CTLA4) therapies that serve to release the inhibition and reactivate cytotoxic T cells (Figure 1C). In this light, immunotherapy has revolutionized the treatment of several cancers, with the highest clinical efficacy seen in melanoma and lung cancer[20]. Immunotherapy has also shown impressive results, in the context of CRC, specifically for dMMR and MSI-high CRC [21]. Since prognosis for late-stage MSI-H CRC is poor, immunotherapy has gained accelerated FDA-approval for several single-agent and combination immunotherapies over the past few years (Table 1). Several more are underway and are being evaluated in large clinical trials[21, 22] to combat refractory cases of CRC.
Figure 1.
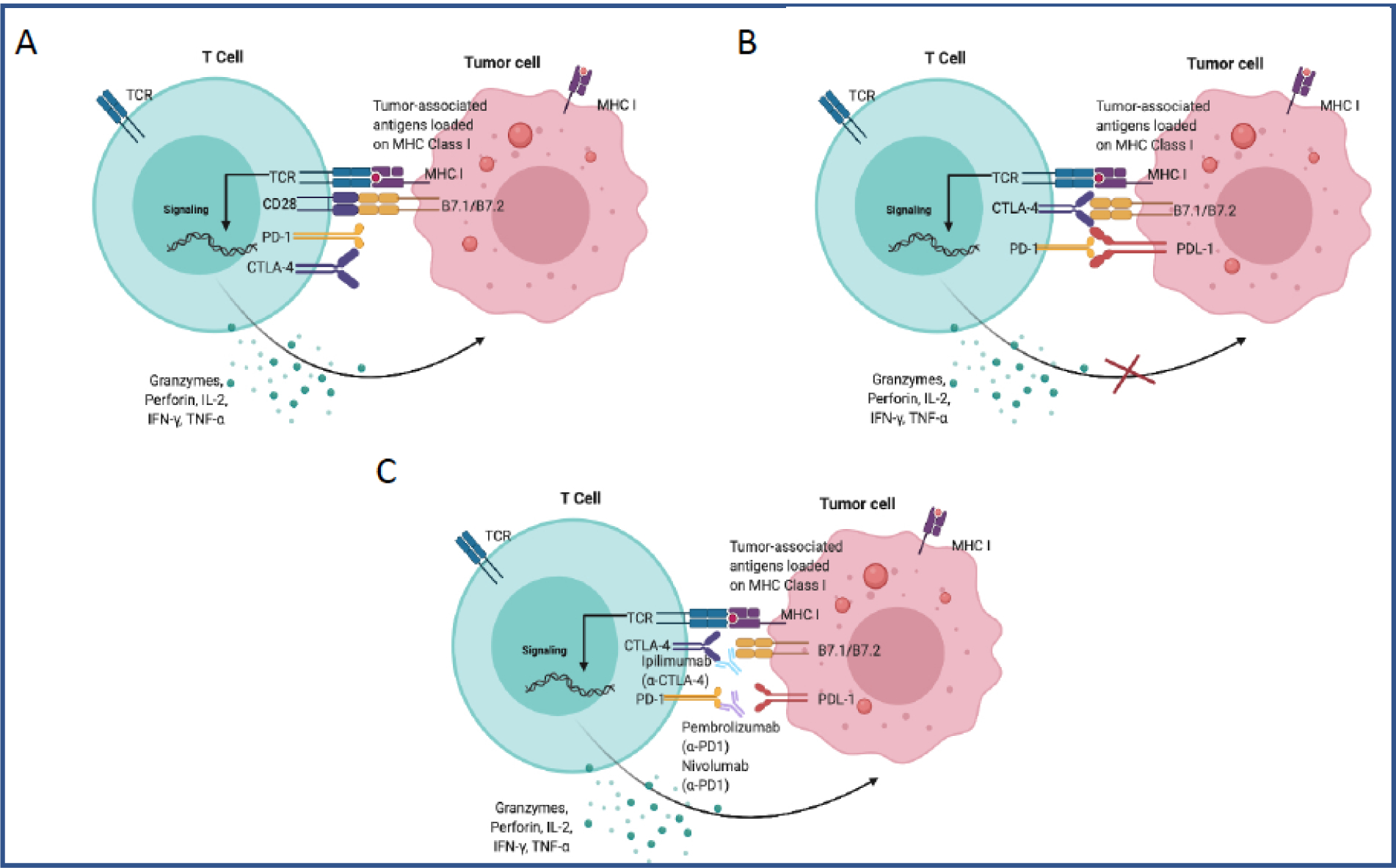
T-cell activation in the tumor microenvironment. A) CD8 T-cells recognizing tumor-associated antigens expressed on MHC class I on tumor cells, receiving co-stimulation from the interaction between CD28 and B7 molecules and resulting in the cytotoxic killing of the tumor cells via release of granzymes and perforins. The inhibitory receptors PD-1 and CTLA4 are not engaged with tumor-cells. B) Immune evasion by tumor cells by expressing inhibitory ligand PDL-1, which binds to PD-1 on T cells, and B7 molecules, which bind to CTLA4. Engagement with inhibitory ligands prevents the cytotoxic killing of tumor cells. C) Blockade of inhibitory receptors ligands interactions with a-PD1 and a-CTLA4 monoclonal antibodies, which results in the restoration of T cell function (Created with BioRender.com).
Table 1.
Clinical Trials results showing the efficacy of single-agent or combination immunotherapy on different types of CRCs
| Clinical Trial | Mutation Status | Intervention | n | ORR | PFS (12 mo) | Median PFS mo | OS (12 mo) | |
|---|---|---|---|---|---|---|---|---|
| Neoadjuvant Immunotherapy | NCT03026140 [23] | Early stage dMMR | Nivolumab+ Ipilimumab | 7 | 57% | NE | NE | NE |
| Single agent Immunotherapy | NCT02060188 CM-142[21] | dMMR/ MSI-H | Nivolumab | 74 | 31% | 50% | 14.3 | 73% |
| NCT02628067 KN-164[24] | dMMR/ MSI-H | Pembrolizumab | 61 | 28% | 34% | 2.3 | 72% | |
| NCT02563002 KN-177[25] | dMMR/ MSI-H | Pembrolizumab First-line | 307 | 43.8% | 55.3% | 16.5 | NE | |
| Combination Immunotherapy | NCT02060188 CM-142[26] | dMMR/ MSI-H mCRC Or BRAF/K RAS WT or mutant | Nivolumab + Ipilimumab | 119 | 55% | 71% | NR | 85% |
| CM-142[27] | MSI-H/dMMR mCRC | Nivolumab + Ipilimumab (low-dose) | 45 | 60% | 77% | NR | 83% | |
| REGONIVO[28] | 98% MSS | Regorafenib+ Nivolumab | 50 | 36% | NE | 7.9 | NE |
NR=Not Reached
NE=Not Evaluated
PD1 inhibitor: Pembrolizumab
PD-1 expression on the surface of T cells is a common mechanism of immune regulation. Within tumor-microenvironment, tumors evade the immune response by expressing PD-1 ligand (PDL1), which acts to “inactivate” the infiltrating cytotoxic T cells. Therefore, treatment with anti-PD1 or anti-PDL1 monoclonal antibodies involves blocking PD-1/PDL-1 interaction and thereby relieving immunosuppression and enhancing cytotoxicity. In a tissue microarray, CRC tumors were found to have high PDL-1 expression in 5% of cases, whereas PD-1 expression on T cells in 19% of cases. Further categorizing the tumors by MMR status, the dMMR tumors were found to have significantly more PD1 (18% in dMMR versus 2% in pMMR) and higher PDL1 expression (50% in dMMR versus 13% in pMMR) compared to pMMR tumors[29]. In a phase II trial conducted to evaluate the clinical efficacy of Pembrolizumab, a humanized IgG4 monoclonal antibody targeting PD1, the clinical benefit of PD-1 blockade was found to correlate with mismatch repair status of the patients, with MSI-high patients more likely to respond [22].
The immune-potentiating effect of PD1 blockade was then tested in a larger phase II clinical trial (KEYNOTE-164), where the anti-tumor activity of Pembrolizumab in previously treated metastatic, dMMR and MSI-high CRC was evaluated[30, 24]. In a cohort of CRC patients aged >- 18 years who have had metastatic MSI-high CRC and were treated with >−2 prior lines of standard therapy, including fluoropyrimidine, oxaliplatin, and irinotecan (FOLFOXIRI) with or without anti-vascular endothelial growth factor/epidermal growth factor receptor monoclonal antibody (cohort A) or >1 prior line of therapy (cohort B), administration of Pembrolizumab led to an objective response rate (ORR) of 28–33% in both cohorts, median progression-free survival (PFS) of 2.3–4.1 months, a 12-month PFS of 34% and a 12-month overall survival(OS) of 72% (Table 1). Remarkably, recent KEYNOTE-158 trial found tumor-mutational burden (TMB)-high MSS solid tumors associated with higher ORR (28.3% for TMB-high MSS compared to 6.5% for TMB-low MSS) in patients treated with Pembrolizumab[31]. These findings suggest that both MSI-high and MSS CRC can benefit from anti-PD1 monotherapy. It is likely that high MSI and tumor-mutational burden generate neoantigens, and promote immunosurveillance. Other anti-PD1 monoclonal antibodies are also being evaluated for both single as well as combination therapy for MSI-high CRC and are discussed below. Pembrolizumab received accelerated approval by the FDA in May 2017[32] for unresectable or MSI-high or mismatch repair deficient (dMMR) metastatic CRC.
The recent completion of phase III KEYNOTE-177 trial evaluating the antitumor activity of Pembrolizumab compared to standard chemotherapy as first-line therapy for patients with MSI-H/dMMR mCRC has revealed some exciting results (Table 1). When used as front-line therapy for MSI-H/dMMR mCRC, Pembrolizumab doubled PFS compared to chemotherapy (16.5months with Pembrolizumab vs. 8.2 months with chemotherapy)[25] (Table 1). Furthermore, the response also lasted longer, with 83% patients on Pembrolizumab showing response longer than 2 years, compared to 35% of patients receiving chemotherapy. While the efficacy of Pembrolizumab was apparent from earlier non-randomized trials, demonstrating its efficacy as first-line treatment in randomized phase-III trial has further highlighted its benefit, establishing Pembrolizumab as the new standard of care for MSI-H/dMMR mCRC patients. Whether Pembrolizumab has potential to perform at even higher efficacy as first-line combination therapy needs to be assessed.
PD-1 inhibitor: Nivolumab
Another anti-PD-1 monoclonal antibody that has shown efficacy in clinical trials is Nivolumab. The efficacy of Nivolumab in CRC was assessed in a phase II trial (Checkmate-142), which saw a 31% ORR in adults aged >−18 year with histologically confirmed recurrent or metastatic CRC locally assessed as MSI-high from 31 sites in eight countries[21]. The study found that similar to Pembrolizumab, Nivolumab provided a durable response in patients who had already progressed to an advanced stage or had been tolerant of the previous line of treatment, including fluoropyrimidine and oxaliplatin and irinotecan. 51 of 74 patients enrolled in the trial showed disease control for 12 weeks or longer, with another 8 showing response that lasted for 12 months or longer. Compared to Pembrolizumab (when used as second-line therapy), the median PSF for Nivolumab was longer (14.3 months), and 12-month PSF was higher (50%). 12-months OS was, however, was comparable at 73% (Table 1).
In a Japanese phase I study, Nivolumab was shown to be safe even at high dose (20mg/kg) in solid malignant tumors, including CRC, with treatment-related grade 3 or 4 adverse occurring in only 11.8% of enrolled patients[33]. These findings suggest that Nivolumab has comparable efficacy and safety profile to Pembrolizumab, and more importantly, comparable or higher overall response rate and overall survival rate compared to Pembrolizumab. As both Checkmate-142 and KEYNOTE-164 trials incorporated patients from sites around the world, any genetic determinant associated with the response to the therapy is less likely. Nivolumab was granted accelerated approval by the FDA in August 2017, immediately following Pembrolizumab, for unresectable or MSI-high or mismatch repair deficient (dMMR) metastatic CRC.
Combination therapy with Nivolumab (PD-1 inhibitor) and Ipilimumab (CTLA-4 inhibitor)
The immune system has multiple immune regulators that function to keep immune activation in check. One of these molecules expressed on the surface of T cells is CTLA-4. CTLA-4 is expressed in several tumor-infiltrating T cells and contributes to immune exhaustion. Due to the clinical efficacy of anti-PD1 monotherapy, several combination therapy trials with anti-CTLA-4 antibodies (Ipilimumab) are now being evaluated for any synergistic effect. Compared to monotherapy, combination therapy with Nivolumab and low-dose Ipilimumab has demonstrated enhanced clinical efficacy and safety [34, 27]. As shown in Table 1, of 119 MSI-high CRC patients refractory to chemotherapy and receiving Nivo+ low-dose IPI reached an ORR of 55% (95% CI, 45.2,63.6), progression-free survival (PFS) rates of 76% at 9 months and 71% at 12 months and overall survival rates (OS) of 87% at 9 months and 85% at 12 months[26]). Treatment-related grade 3–4 adverse events were found in 32% of patients and were manageable. Furthermore, toxicity was reported in the early 3 months of treatment but showed reduced toxicity once the 3-month landmark was crossed. Analyses from the trial showed that the combination produced similar results both when used in frontline (ORR, 55% (95% CI, 45.2%−63.8%) or in patients with refractory disease (ORR, 60%; 95% CI, 44%−74%).
Overall, Nivolumab and Ipilimumab combination therapy demonstrates high response rates, enhanced clinical benefit, and manageable safety compared to monotherapy, and therefore, provides a promising treatment option for MSI-high CRC. Nivolumab with low-dose Ipilimumab is the first immuno-oncology combination drug approved for dMMR/MSI-H metastatic CRC patients who were refractory to treatment with standard chemotherapy.
Other immunotherapy monoclonal antibodies
Other antibodies used for PD1/PDL-1 blockade include Atezolizumab, which is a humanized IgG1 molecular antibody that blocks PD-L1 and helps inhibit the PD-1/PDL-1 axis. Results from clinical trials have shown none to limited drug-related toxicities (Table 2). Similarly, Avelumab is another anti-PD-L1 blocking antibody, which is being evaluated in combination with chemotherapy (Table 2).
Table 2.
Prior and ongoing clinical trials investigating the efficacy of combination immunotherapy for different CRC subtypes and stages.
| Clinical Trials | Arms and Interventions | Trial Phase: CRC type | Trial Registration Number | Date of Registration |
|---|---|---|---|---|
| CheckMate-142 | Nivolumab alone Nivolumab+Ipilimumab Nivolumab+Ipilimumab+Cobimetinib Nivolumab+BMS-986016 (anti-LAG3) Nivolumab+Daratumumab |
Phase II: MSI CRC MSS CRC dMMR CRC pMMR CRC MSI positive or negative recurrent/mCRC |
NCT02060188 | 2014 |
| Pembrolizumab | Phase II: MSI +ve CRC MSI-ve CRC MSI +ve non CRC |
NCT01876511 | 2013 | |
| MK-3475–164/KEYNOTE-164 | Pembrolizumab | Phase II: Previously treated locally advanced or mCRC | NCT02460198 | 2015 |
| KEYNOTE-177 | Pembrolizumab | Phase III: MSI-H/dMMR mCRC first-line therapy | NCT02563002 | 2015 |
| KEYNOTE-158 | Pembrolizumab | TMB-high solid tumors | NCT02628067 | 2015 |
| Pembrolizumab+MK-8353 | Phase II: Advanced CRC | NCT02972034 | 2016 | |
| COMMIT study | FOLFOX/bevacizumab FOLFOX/bevacizumab/atezolizumab |
Phase III: MSI-H mCRC | NCT02997228 | 2016 |
| ATOMIC, Alliance A021502 | Atezolizumab + Chemotherapy (FOLFOX) | Phase III: Stage III CRC and dMMR CRC | NCT02912559 | 2016 |
| AVETUXIRI | Avelumab+Cetuximab+Irinotecan | Phase II: Refractory MSS mCRC | NCT03608046 | 2018 |
| Multiple Immunotherapy Combinations including Atezolizumab, Regorafenib, Imprime PGG, Bevacizumab, Isatuximab, Selicrelumab, Idasanutlin, AB928 | Phase I and II: mCRC refractory to first and second-line therapy | NCT03555149 | 2018 | |
| M7824 (anti-PDL1/TGFβRII fusion protein) | Phase I and II: MSI advanced CRC | NCT03436563 | 2018 | |
| CAMILLA | Durvalumab+ Cabozantanib | Phase II: pMMR/MSS mCRC | NCT03539822 | 2018 |
| REGONIVO | Nivolumab+ Regorafenib in Combination with Radiotherapy | Phase II: pMMR/MSS mCRC | NCT04030260 | 2019 |
| MAYA | Nivolumab+Ipilimumab+ Temozolomide | Phase II: MSS, MGMT silenced mCRC | NCT03832621 | 2019 |
| CAROSELL | N ivolumab+CXD 101 | Phase I and II: MSS mCRC | NCT03993626 | 2019 |
| Nivolumab+Encorafenib+Binimetinib | Phase I and II: MSS BRAFV600E mCRC | NCT04044430 | 2019 | |
| Nivolumab+Encorafenib+ Cetuximab | Phase I and II: MSS, BRAFV600E unresectable or mCRC | NCT04017650 | 2019 | |
| Nivolumab+Metformin | Phase II: Refractory MSS CRC | NCT03800602 | 2019 | |
| Nivolumab+ Circulating tumor DNA | Phase III: Stage III CRC | NCT03803553 | 2019 | |
| Pembrolizumab Nivolumab Atezolizumab Ipilimumab | Phase III: Advanced CRC | NCT04157985 | 2019 |
BRAF mutation in MSI-high: efficacy of immunotherapy
The efficacy of immunotherapy appears to rely not only on MSI status but also on pre-existing gene mutations. BRAF-V600E mutation is known to have a poor prognosis for metastatic CRC patients. A strong correlation has been described between BRAF mutation and aberrant DNA methylation of promoter or CpG islands (also called CpG-island methylator phenotype), which is associated with gene silencing[35]. One of the important genes silenced by promoter hypermethylation in this context is MLH1, which encodes for a mismatch repair protein. Microsatellite instability resulting from MLH1 hypermethylation, together with BRAF mutation, puts this CRC into the CMS1 subtype. A study reported that compared to MSS/BRAF WT subtype, MSI/BRAF-V600E subtype had a favorable prognostic factor[36]. Whether prognosis associated with BRAF mutation can be further improved by checkpoint blockade needs to be examined. A clinical trial assessing the efficacy of combination therapy with Nivolumab and low-dose Ipilimumab also evaluated the BRAF mutation status of the participants and found that the overall response rate (55%) remained constant, irrespective of their BRAF mutation status[27] (Table 1). Furthermore, no difference between the disease control rate was noted between the groups. Similar results were obtained with Nivolumab monotherapy, therefore, suggesting that BRAF mutation does not have predictive value in foreseeing the efficacy of anti-PD1 and anti-CTLA-4 checkpoint blockade.
Immunotherapy for resected CRC patients in the adjuvant setting
Immunotherapy has not only been evaluated for the late-stage disease but also for early-stage CRCs. Histological and clinical evaluation of stage II CRC has demonstrated marked heterogeneity among several stage II CRCs, and recurrence after curative surgical resection. Therefore, there has been increasing effort in evaluating immunotherapy for different subsets of early-stage CRCs. Stage I and stage II CRCs with high immunoscore have been reported to have a 5-year OS of 86.2%, whereas those with low immunoscore have been reported to have a 5-year OS of 27.5%[37]. This finding was further validated by another study, which reported that immunoscore could predict disease-free survival and OS in stage II CRC and, more importantly, was able to identify the subset of CRC with lower versus a higher rate of recurrence[38]. More recently, evaluating short-term neoadjuvant Ipilimumab plus Nivolumab prior to surgery for dMMR showed a 100% response rate and did not compromise surgery[23]. The use of immunoscore in predicting subsets of stage II CRC patients responsive to immunotherapy could have great potential in CRC prognosis and treatment.
Vaccines with Immunotherapy
Tumor vaccine is another aspect of immunotherapy that holds promise in eliciting a durable anti-tumor response. In the context of CRC, guanylyl cyclase C(GUCY2C), a membrane-spanning receptor for the synthesis of cyclic GMP (cGMP), has been shown to be universally over-expressed by primary and recurrent colorectal cancer and has been incorporated into a vaccine for CRC[39]. GUCY2C has been detected in almost 1000 CRC specimens, but not in extra-gastrointestinal tissues or tumors. Furthermore, even within intestinal epithelial cells, GUCY2C shows localization in the apical brush border membrane and, therefore, away from the mucosa. This adenovirus (Ad5)-delivered GUCY2C-based vaccine was tested in ten patients with surgically resected stage I or stage II CRC patients for memory CD8+ T cell response. The study resulted in antibody response to GUCY2C in 10% of patients, whereas GUCY2C-specific T cell response in 40% of patients[39]. More importantly, T-cell response was noted to be primarily CD8 T cell response, and not CD4-based respond, suggesting minimal autoimmune response.
Vaccine attempts have also been made for metastatic CRCs. The vaccine candidate tested for mCRC in phase I trial is PolyPEP1018, which is a peptide vaccine that contains 12 unique epitopes from seven conserved sites from cancer testis antigens (CTAs), which are frequently expressed in mCRC[40]. Evaluation of 4/11 patients receiving the vaccine demonstrated T-cell response by 96% of peptides, with 2 patients showing unexpected reduction in tumor size. Furthermore, the vaccine was found to be safe and well-tolerated. These early promising results have led to amendment of the trial as well as preparation for a larger trial.
Role of Microbiome with Immunotherapy
As discussed in earlier sections, molecular biomarkers of CRC play an important role in the staging and prognosis of CRC, and in predicting the efficacy of immunotherapy. In this context, the gut microbiome has also been emerging as a potential determinant. The potential role of the microbiome in the efficacy of immunotherapy has been studied in more depth in melanoma and less so in CRC. In a recent study, twenty-five metastatic melanoma patients were evaluated for their gut microbiome composition before and after treatment with Ipilimumab, demonstrating that the efficacy was correlated with an increase in abundance of Bacteroides species[41]. Transplantation of feces harvested from individual patients into tumor-bearing germ-free mice that were subsequently treated with Ipilimumab demonstrated that the tumor-bearing mice transplanted with feces from Bacteroides dominant sample responded well to CTLA-4 blockade, in contrast to those receiving feces containing other bacterial species.
Based on these findings, studies have investigated if gut microbial composition also has a role in CRC. A recent study reported a consortium of 11 bacterial strains from healthy human donor feces, which were capable of inducing robust CD8-T cell response in mouse intestine without causing inflammation[42]. Furthermore, the 11 strains that represent the low-abundance component of the human microbiome enhanced the therapeutic efficacy of immune checkpoint inhibitors upon colonizing a syngeneic mouse tumor model. In another study, adoptive transfer of human tumor-infiltrating lymphocytes from CRC into tumor-bearing mice was found to enhance expression of chemokines, which dramatically reduced upon antibiotic treatment, suggesting that microbiome stimulate chemokine production by CRC cells, thereby favoring the recruitment of T cells and potentially the efficacy of immune checkpoint blockade[43]. Enrichment of Fusobacteria and Bacteriodetes, and concurrent reduction in Firmicutes and Proteobacteria have been reported in MSI and dMMR, hypermutated CRC with BRAF mutation[44, 45]. With these findings, it is apparent that consideration for microbiome composition as a marker for different subsets of CRC may help enhance the efficacy of immunotherapy even further. In this context, alteration of patients’ microbiota into “favorable” microbiome via fecal microbial transplant (FMT) could be a strategy to maximize the efficacy of immunotherapy[46].
MSS Colorectal Cancer: Chemotherapy, immunomodulators, and immunotherapy
One of the exciting aspects surrounding the use of biologics or immunotherapy for the treatment of colorectal cancer is their markedly reduced toxicity compared to the standard chemotherapy. Unfortunately, immunotherapy has not been very efficacious for microsatellite stable (MSS) or MMR proficient (pMMR) CRC, which makes a vast majority of CRC cases. No meaningful benefit has been noted for MSS CRC with either single-agent PD-1 or combination of PD-1 and CTLA-4 blockade[47]. However, recent KEYNOTE-158 trial demonstrated that MSS solid tumors (non-CRC) do respond to anti-PD1 therapy (Pembrolizumab) when they harbor high tumor-mutational burden (TMB), associated with higher ORR and favorable PFS [31]. In the context of CRC, nearly 3% of CRC have been identified as MSS/TMB-high[48]. This finding suggests that stratifying MSS mCRC patients by tumor-burden may help broaden the coverage of immunotherapy.
Among immunomodulators, VEGF-A has been shown to modulate the expression of inhibitory receptors in CD8+ T cells in tumors and therefore has an important role in regulating the immunosuppressive environment [49]. Enhanced activation of TGF beta pathways is characteristic of the CMS4 subtype of CRC and is associated with less mutation-burden, microsatellite stability and tumor infiltration. One potential mechanism to target CMS4 would be to inhibit the inactivating effects of VEGF signaling and enhance immunogenicity with immunotherapy. Several clinical trials (e.g., NCT01633970, NCT02873195, NCT02876224) are investigating whether bevacizumab, a recombinant humanized monoclonal antibody against VEGF-A alone or in combination with Atezolizumab (anti-PDL1) is efficacious in metastatic MSS CRC.
The other two metastatic microsatellite stable (MSS) CRCs are CMS2, and CMS3, which are designated as “cold tumors,” and are characterized as lacking immune infiltrates [50]. Due to a lack of immune involvement, immunotherapy alone has not been found to provide any clinical benefits for these CRC subtypes. Since these subsets of CRC constitutes a large fraction of metastatic CRC cases, an ongoing attempt has been to identify disease pathways that can be targeted in synergy with immunotherapy. Interestingly, in a cohort study involving type II diabetic patients, long-term metformin use was associated with a reduced risk of developing CRC[51]. This effect was attributed to the activation of AMP-activated protein kinase (AMPK) by metformin, known to inhibit cell proliferation, angiogenesis, fatty acid synthesis, and induction of cell cycle arrest, autophagy, and apoptosis. Currently, metformin is being evaluated for combination therapy with Nivolumab in treating patients with microsatellite stable (MSS) stage IV CRC that has not responded to previous treatment (NCT03800602).
Almost 85% of patients with metastatic CRC are MSS. Several attempts at treating this subset of CRC with single-agent immunotherapy treatment have been unsuccessful. One attempt to target MSS CRC has been to combine immunotherapy with chemotherapy. Combination therapies being tested for refractory microsatellite stable (MSS) CRC include Nivolumab plus Ipilimumab with temozolomide, which is an alkylating agent (NCT03832621). Since most early-stage CRC lack immunogenicity and demonstrate microsatellite stability, one approach has been to examine combination therapy in earlier stages of CRC. A recent phase II clinical trial assessed the efficacy of Nivolumab plus Ipilimumab in early-stage dMMR and pMMR (MMR proficient) colon cancers [23]. Upon treatment, 100% showed a major pathological response in dMMR, quantified as < 5% viable tumors, and 57% showed complete response. Although no pathological response was noted for pMMR, there was a significant increase in T-cell infiltration, specifically of CD8+ T cells post-treatment in both pMMR and dMMR cases, with a median fold change of 2.4 (p=0.018) and 4.8 (p=0.0009), respectively. These findings suggest that there might be potential for neoadjuvant Nivolumab and Ipilimumab therapy for MSS or pMMR CRCs in early disease.
Similarly, there are ongoing clinical trials investigating the efficacy of combination therapy including, multikinase inhibitor regorafenib (Stivarga) and anti-PD1 immune checkpoint inhibitor Nivolumab (Opdivo) in the treatment of microsatellite stable (MMS) metastatic CRC. REGONIVO trial conducted in a Japanese cohort included 50 patients with previously treated advanced or metastatic gastric or colorectal cancer[28]. With the recommended dose of regorafenib, the overall response rate was 36% for CRC, of which all but 1 had MSS CRC (Table 1). Furthermore, progression-free survival was found to be 6.3 months. Regorafenib has now been FDA approved for the treatment of previously treated refractory mCRC patients. With proven efficacy and safety of regorafenib, combination therapy with regorafenib and Nivolumab is now being evaluated on a larger scale.
On-going clinical trials
Table 2 chronologically summarizes prior and ongoing single and combination trials investigating the safety and efficacy of immunotherapy for several subtypes and stages of CRCs. As can be seen, most earlier trials focused on MSI and dMMR CRC subtypes. However, increasing response rates observed with MSS and pMMR CRC due to combined immunotherapy has led to a dramatic increase in the number of MSS-focused clinical trials in recent years.
Conclusion
Immunotherapy holds great promise in guiding future interventions. Approved use of Pembrolizumab as first-line therapy for MSI-high/dMMR mCRC suggests that immunotherapy can change the standard of care for this CRC subtype. While single-agent immunotherapy has been demonstrated to be superior to chemotherapy in treating MSI-high/dMMR mCRC, combination therapy has further improved responsiveness and overall survival. In recent trials, immunotherapy has also shown its benefits for MSS and TMB-high solid tumors, highlighting potential for broadening immunotherapy to CRC subsets with these features. Considering these improvements, it is of no surprise that in the future, several other T cell modulators as well as biologics will be evaluated to treat different subtypes of CRCs.
Apart from MSI status, gene-specific mutations (eg BRAF, NRAS etc) as well as tumor-mutational burden are also emerging as valuable predictors of immunotherapy efficacy. One of the poor prognostic features of CRC for chemotherapy is the presence of BRAF-V600E mutation. However, for immunotherapy, BRAF mutation status alone appears to be less significant in predicting its effectiveness. Whether the mutation status of other genes such as KRAS, APC, p53, PI3K and so forth make good predictors needs to be evaluated. On the other hand, high tumor-mutation burden does seem to enhance efficacy and needs further validation. Similarly, factors such as the composition of gut microbiota and cancer vaccines are promising and have the potential to make an impact. Overall, understanding the molecular features, mutation status, tumor-mutational burden and microbiome profile of patients and making an appropriate choice of single versus combination therapy agents has important implications in directing future care.
Acknowledgments
The editors would like to thank Dr. Gopichand Pendurti for taking the time to review this article.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
Sophiya Karki, Shahid Umar, and Anup Kasi each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Recently published papers of particular interest have been highlighted as:
● Of importance
●● Of major importance
- 1.American Cancer Society. Cancer Statistics Center. Available at: https://cancerstatisticscenter.cancer.org/#/cancer-site/Colorectum. Accessed January 25, 2020 2020.
- 2.Angell HK, Bruni D, Barrett JC, Herbst R, Galon J. The Immunoscore: Colon Cancer and Beyond. Clin Cancer Res. 2020;26(2):332–9. doi: 10.1158/1078-0432.Ccr-18-1851. [DOI] [PubMed] [Google Scholar]
- 3.Reimers MS, Zeestraten EC, Kuppen PJ, Liefers GJ, van de Velde CJ. Biomarkers in precision therapy in colorectal cancer. Gastroenterol Rep (Oxf). 2013;1(3):166–83. doi: 10.1093/gastro/got022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts & Figures 2020. Atlanta: American Cancer Society;. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokolenko AP, Imyanitov EN. Molecular Diagnostics in Clinical Oncology. Front Mol Biosci. 2018;5:76-. doi: 10.3389/fmolb.2018.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.●.Okita A, Takahashi S, Ouchi K, Inoue M, Watanabe M, Endo M et al. Consensus molecular subtypes classification of colorectal cancer as a predictive factor for chemotherapeutic efficacy against metastatic colorectal cancer. Oncotarget. 2018;9(27):18698–711. doi: 10.18632/oncotarget.24617 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study assessed CMS subtypes of CRC for disease prognosis and therapeutic efficacy against mCRC and has had important implication in designing immunotherapy trials.
- 7.Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–6. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becht E, de Reynies A, Giraldo NA, Pilati C, Buttard B, Lacroix L et al. Immune and Stromal Classification of Colorectal Cancer Is Associated with Molecular Subtypes and Relevant for Precision Immunotherapy. Clin Cancer Res. 2016;22(16):4057–66. doi: 10.1158/1078-0432.Ccr-15-2879. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Salas N, Dominguez G, Barderas R, Mendiola M, Garcia-Albeniz X, Maurel J et al. Clinical relevance of colorectal cancer molecular subtypes. Crit Rev Oncol Hematol. 2017;109:9–19. doi: 10.1016/j.critrevonc.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Peltomaki P Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol. 2003;21(6):1174–9. doi: 10.1200/jco.2003.04.060. [DOI] [PubMed] [Google Scholar]
- 11.Burt R Inheritance of Colorectal Cancer. Drug Discov Today Dis Mech. 2007;4(4):293–300. doi: 10.1016/j.ddmec.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grady WM, Pritchard CC. Molecular alterations and biomarkers in colorectal cancer. Toxicol Pathol. 2014;42(1):124–39. doi: 10.1177/0192623313505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology. 2010;138(6):2059–72. doi: 10.1053/j.gastro.2009.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 15.Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25(2):261–7. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 16. ●.Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128–39. doi: 10.1016/s0140-6736(18)30789-x [DOI] [PubMed] [Google Scholar]; This study highlights the importance of consensus immunoscore to aid TNM-immune classification system of cancer.
- 17.Koopman M, Kortman GA, Mekenkamp L, Ligtenberg MJ, Hoogerbrugge N, Antonini NF et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer. 2009;100(2):266–73. doi: 10.1038/sj.bjc.6604867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jo W-S, Carethers JM. Chemotherapeutic implications in microsatellite unstable colorectal cancer. Cancer Biomark. 2006;2(1–2):51–60. doi: 10.3233/cbm-2006-21-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papaioannou NE, Beniata OV, Vitsos P, Tsitsilonis O, Samara P. Harnessing the immune system to improve cancer therapy. Ann Transl Med. 2016;4(14):261-. doi: 10.21037/atm.2016.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huyghe N, Baldin P, Van den Eynde M. Immunotherapy with immune checkpoint inhibitors in colorectal cancer: what is the future beyond deficient mismatch-repair tumours? Gastroenterology Report. 2019. doi: 10.1093/gastro/goz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. ●●.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–91. doi: 10.1016/s1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]; Study that first showed durable response and efficacy of Nivolumab in pre-treated patients with dMMR/MSI-H mCRC.
- 22. ●●.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study looked at 12 different tumor types and described MMR deficiency as a potential biomarker for predicting sucessfull treatment outcomes for these tumors including colorectal cancer.
- 23. ●●.Chalabi M, Fanchi LF, Van den Berg JG, Beets GL, Lopez-Yurda M, Aalbers AG et al. Neoadjuvant ipilimumab plus nivolumab in early stage colon cancer. Annals of Oncology. 2018;29(suppl_8). doi: 10.1093/annonc/mdy424.047 [DOI] [Google Scholar]; This study highlighted potential advantage of using neoadjuvant ipilumumab and nivolumab for early stage CRC.
- 24.Diaz LA, Marabelle A, Delord J-P, Shapira-Frommer R, Geva R, Peled N et al. Pembrolizumab therapy for microsatellite instability high (MSI-H) colorectal cancer (CRC) and non-CRC. Journal of Clinical Oncology. 2017;35(15_suppl):3071-. doi: 10.1200/JCO.2017.35.15_suppl.3071. [DOI] [Google Scholar]
- 25. ●●.Pembrolizumab Doubles Time to Disease Progression in Patients With Advanced Colorectal Cancer With Specific DNA Mutations [press release]. Available at https://www.asco.org/about-asco/press-center/news-releases/pembrolizumab-doubles-time-disease-progression-patients Accessed on June 2, 2020; Phase III KEYNOTE-177 trial showing enhanced efficacy of Pembrolizumab compared to chemotherapy as first-line therapy for MSI-high/dMMR mCRC.
- 26. ●●.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36(8):773–9. doi: 10.1200/jco.2017.76.9901 [DOI] [PubMed] [Google Scholar]; Study showing enhanced efficacy of combination therapy with Nivolumab and Ipilimumab compared to mono therapy with Nivolumab for dMMR/MSI-H mCRC.
- 27.Lenz HJJ, Van Cutsem E, Limon ML, Wong KY, Hendlisz A, Aglietta M et al. Durable clinical benefit with nivolumab (NIVO) plus low-dose ipilimumab (IPI) as first-line therapy in microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC). Annals of Oncology. 2018;29:viii714. doi: 10.1093/annonc/mdy424.019. [DOI] [Google Scholar]
- 28. ●.Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38(18):2053–61. doi: 10.1200/jco.19.03296 [DOI] [PubMed] [Google Scholar]; This study reported first safety and efficacy profile of combination therapy with nivolumab and Regorafenib, a potent inhibitor of angiogenic and oncogenic kinsases for MSS CRC, which makes large proportion of CRC cases.
- 29.Lee LH, Cavalcanti MS, Segal NH, Hechtman JF, Weiser MR, Smith JJ et al. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol. 2016;29(11):1433–42. doi: 10.1038/modpathol.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.●●.Le DT, Kim TW, Van Cutsem E, Geva R, Jager D, Hara H et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J Clin Oncol. 2020;38(1):11–9. doi: 10.1200/jco.19.02107 [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the promising clinical trials that showed efficacy and safety of Pembrolizumab against heavily pretreated patients with MSI-H CRC.
- 31. ●●.Marabelle A, Fakih MG, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K et al. 1192O - Association of tumour mutational burden with outcomes in patients with select advanced solid tumours treated with pembrolizumab in KEYNOTE-158. Annals of Oncology. 2019;30:v477–v8 [DOI] [PubMed] [Google Scholar]; This study demonstrated that tumor-mutational burden high solid tumors can be targeted by immunotherapy, and suggest that immuntherapy can have a larger coverage. doi: 10.1093/annonc/mdz253.018. [DOI]
- 32.Stenger M Pembrolizumab in MSI-H or dMMR Solid Tumors: ‘First Tissue/Site-Agnostic’ Approval by FDA. 2018.
- 33.Yamamoto N, Nokihara H, Yamada Y, Shibata T, Tamura Y, Seki Y et al. Phase I study of Nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Invest New Drugs. 2017;35(2):207–16. doi: 10.1007/s10637-016-0411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andre T, Lonardi S, Wong M, Lenz H-J, Gelsomino F, Aglietta M et al. Nivolumab + ipilimumab combination in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): First report of the full cohort from CheckMate-142. Journal of Clinical Oncology. 2018;36(4_suppl):553-. doi: 10.1200/JCO.2018.36.4_suppl.553. [DOI] [Google Scholar]
- 35.Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38(7):787–93. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 36.Seppala TT, Bohm JP, Friman M, Lahtinen L, Vayrynen VM, Liipo TK et al. Combination of microsatellite instability and BRAF mutation status for subtyping colorectal cancer. Br J Cancer. 2015;112(12):1966–75. doi: 10.1038/bjc.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27(35):5944–51. doi: 10.1200/jco.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 38.Lee JJ, Chu E. Adjuvant Chemotherapy for Stage II Colon Cancer: The Debate Goes On. J Oncol Pract. 2017;13(4):245–6. doi: 10.1200/JOP.2017.022178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snook AE, Baybutt TR, Xiang B, Abraham TS, Flickinger JC, Hyslop T et al. Split tolerance permits safe Ad5-GUCY2C-PADRE vaccine-induced T-cell responses in colon cancer patients. Journal for ImmunoTherapy of Cancer. 2019;7(1):104. doi: 10.1186/s40425-019-0576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hubbard JM, Cremolini C, Graham RP, Moretto R, Mitchelll JL, Wessling J et al. A phase I study of PolyPEPI1018 vaccine plus maintenance therapy in patients with metastatic colorectal cancer with a predictive biomarker (OBERTO). Journal of Clinical Oncology. 2019;37(15_suppl):3557-. doi: 10.1200/JCO.2019.37.15_suppl.3557 [DOI] [Google Scholar]
- 41.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–84. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565(7741):600–5. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 43. ●.Cremonesi E, Governa V, Garzon JFG, Mele V, Amicarella F, Muraro MG et al. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut. 2018;67(11):1984–94. doi: 10.1136/gutjnl-2016-313498 [DOI] [PubMed] [Google Scholar]; This study highlighted the importance of microbiota in favorable T-cell recruitment in human CRC, and provides insight for furture CRC research and clinical trials.
- 44.Temraz S, Nassar F, Nasr R, Charafeddine M, Mukherji D, Shamseddine A. Gut Microbiome: A Promising Biomarker for Immunotherapy in Colorectal Cancer. International journal of molecular sciences. 2019;20(17):4155. doi: 10.3390/ijms20174155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamada T, Zhang X, Mima K, Bullman S, Sukawa Y, Nowak JA et al. Fusobacterium nucleatum in Colorectal Cancer Relates to Immune Response Differentially by Tumor Microsatellite Instability Status. Cancer Immunol Res. 2018;6(11):1327–36. doi: 10.1158/2326-6066.Cir-18-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park R, Umar S, Kasi A. Immunotherapy in Colorectal Cancer: Potential of Fecal Transplant and Microbiota-Augmented Clinical Trials. Current Colorectal Cancer Reports. 2020;16(4):81–8. doi: 10.1007/s11888-020-00456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segal N, Saltz L. Translational Considerations on the Outlook of Immunotherapy for Colorectal Cancer. Current Colorectal Cancer Reports. 2015;11. doi: 10.1007/s11888-015-0258-5. [DOI] [Google Scholar]
- 48.Fabrizio DA, George TJ Jr., Dunne RF, Frampton G, Sun J, Gowen K et al. Beyond microsatellite testing: assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition. J Gastrointest Oncol. 2018;9(4):610–7. doi: 10.21037/jgo.2018.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet A-L et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. The Journal of experimental medicine. 2015;212(2):139–48. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roelands J, Kuppen PJK, Vermeulen L, Maccalli C, Decock J, Wang E et al. Immunogenomic Classification of Colorectal Cancer and Therapeutic Implications. Int J Mol Sci. 2017;18(10). doi: 10.3390/ijms18102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bradley MC, Ferrara A, Achacoso N, Ehrlich SF, Quesenberry CP Jr., Habel LA. A Cohort Study of Metformin and Colorectal Cancer Risk among Patients with Diabetes Mellitus. Cancer Epidemiol Biomarkers Prev. 2018;27(5):525–30. doi: 10.1158/1055-9965.Epi-17-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]


