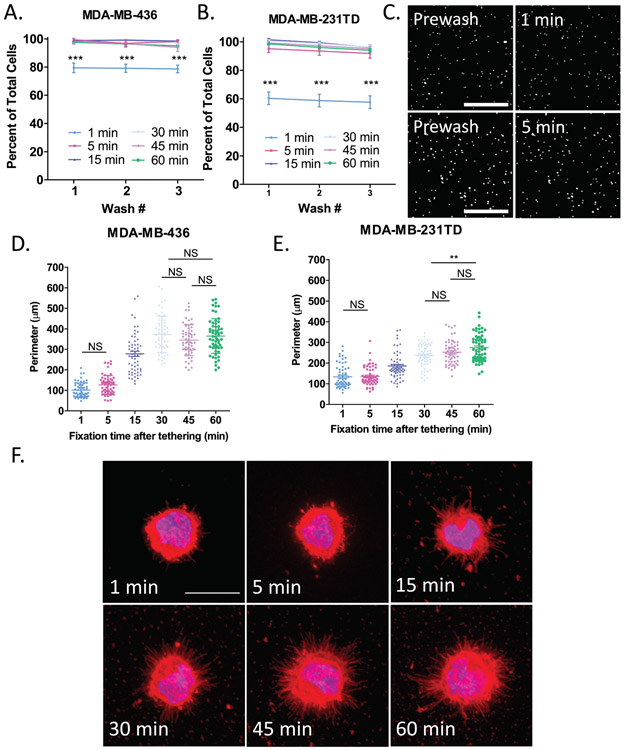
Figure 4: Time course of cell tethering and microtentacle fixation on thermal-crosslinked surface.
A-B. Percent of tethered cell retention after sequentially washing A) MDA-MB-436 or B) MDA-MB-231TD cells on microfluidic slides coated with thermal-crosslinked (PMA/PAAm)1+DOTAP after the indicated time points. Data are shown as mean ± SEM, n=3; ***, P < 0.001 vs. 1 minute (two-way ANOVA with Bonferroni posttest). C) Representative images of tethered MDA-MB-436 cells stained with NucBlue Live ReadyProbes Reagent on microfluidic slides coated with thermal-crosslinked (PMA/PAAm)1+DOTAP either washing after 1 minute (top panel) of tethering time or 5 minutes (bottom panel). Images were taken at 4X magnification in the DAPI channel on the Nikon Eclipse Ti2-E inverted microscope. Scale bar = 500μm. D-E) Quantification of the perimeter of either D) MDA-MB-436 cells or E) MDA-MB-231TD cells at each of the indicated time points analyzed by ImageJ. Data are shown as mean ± SEM, n=3; All multiple comparisons are significant with P < 0.001 unless otherwise stated on graph; **, P < 0.01; NS, P > 0.05 vs. indicated data point (one-way ANOVA with Bonferroni posttest). F) Representative images of tethered MDA-MB-436 cells fixed at either 1, 5 ,15, 30, 45, or 60 min post-tethering and stained with Hoechst 33258 (1:5000) and WGA (1:100). Images were taken at 60X magnification using an Olympus IX81 microscope with a Fluoview FV1000 confocal laser scanning system. Scale bar = 20μm.