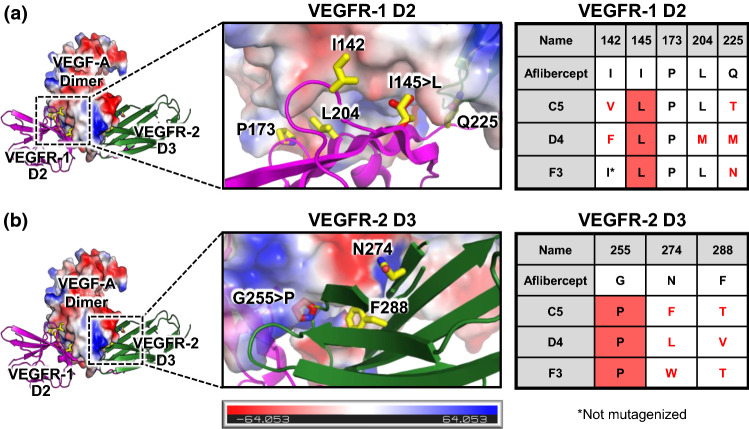
Figure 5.
Sequence convergence of enhanced-affinity aflibercept variants. The molecular structure of the complex between VEGF-A and aflibercept was modeled by overlaying the crystallographic structures of VEGF-A bound to VEGFR-1 D2 (PDB ID: 5T89)29 and VEGF-A bound to VEGFR-2 D3 (PDB ID: 3V2A).5 Enlarged views of the VEGF-A/VEGFR-1 interface (a) and the VEGF-A/VEGFR-2 interfaces (b) are shown. VEGF-A is colored based on an electrostatic map, and residues that were mutagenized in the library are shown in yellow. Predicted orientations of convergently mutated residues are shown in red. Sequences of the engineered aflibercept variants at each mutated position are presented in the table at right. Mutations from the wild type aflibercept residue are indicated with red text and convergent substitutions are indicated with red shading.