Figure 3.
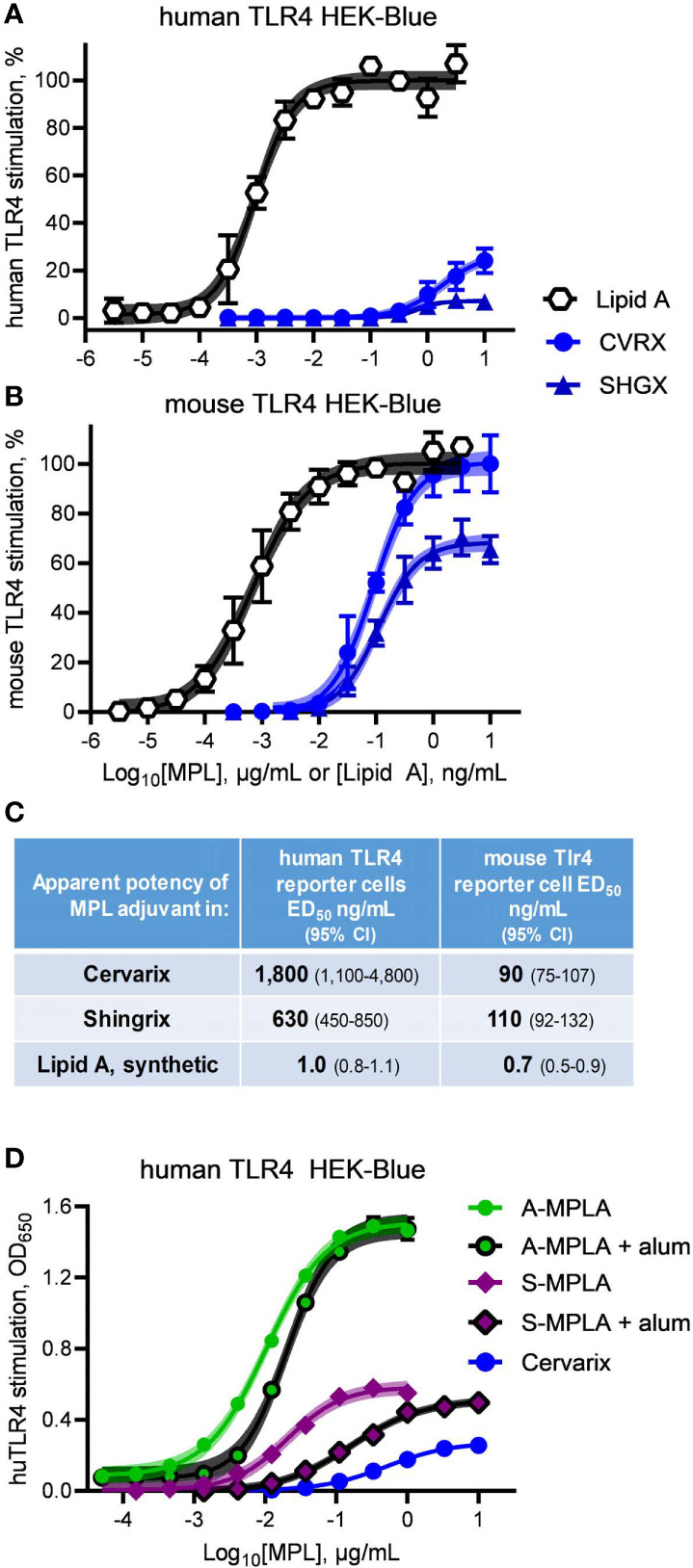
Clinical-grade MPL adjuvant is a partial agonist of human TLR4. Clinical-grade MPL adjuvant® was evaluated for human vs mouse TLR4 dose response profiles by exposing (A) human TLR4 and (B) mTLR4 HEK-Blue reporter cells to Cervarix vaccine (CVRX) or the AS01 adjuvant component of Shingrix vaccine (SHGX) for 24 h. 100% = the top dose plateau for Lipid A-induced secretion of alkaline phosphatase, which was used to normalize TLR4 stimulatory activity between the two reporter cell-lines for a combined total of three independent experiments. (C) MPL adjuvant® was 5–16% as potent an agonist of human TLR4 than it was of mouse TLR4 as determined by stimulatory curve fitting to calculate ED50 for the curves shown in (A, B). Low dose plateaus of human TLR4 responses to MPL adjuvant® relative to Lipid A and mouse TLR4 responses, indicate it is a partial agonist of human TLR4. (D) human TLR4 HEK-Blue cells were treated with MPLA adsorbed on alum and compared to MPLA alone; alum adsorption did not decrease efficacy of two MPLA preparations. Curve fitting to calculate the top dose plateaus with and without alum produced the following values (with 95% confidence intervals): A-MPLA, 1.51 (1.49–1.53) vs A-MPLA + alum, 1.49 (1.47–1.52); S-MPLA, 0.59 (0.57–0.60) vs S-MPLA + alum, 0.51 (0.50–0.53). Shaded regions indicate 99% confidence intervals within which the true population means should occur 99% of the time.
