Figure 4.
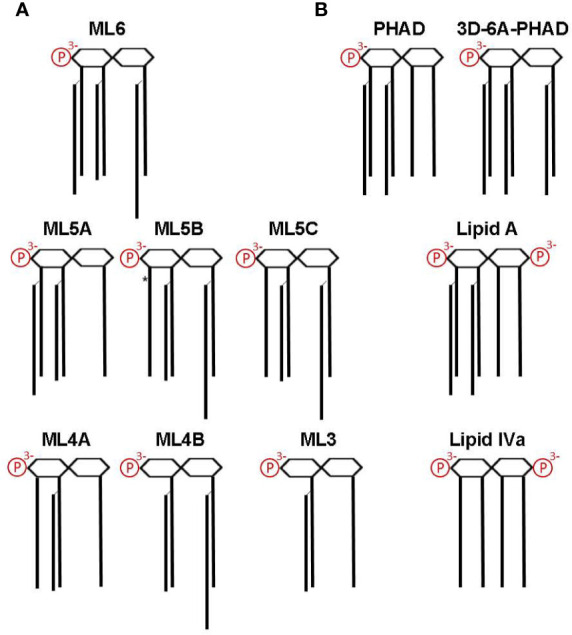
Structures of MPL components and other Lipid A used in this study. Lipid A structures are shown as simplified diagrams representing a β-(1→6) di-glucosamine backbone with varying numbers of acyl chains and phosphates; 1- and 4′ phosphorylation sites are depicted on the right and left, respectively. Amino, ether, ester, and hydroxide linkages are omitted. (A) The major components of MPL adjuvant® are congeners that differ primarily at the level of acyl chain attachment sites and lengths. All MPL® adjuvant components lack an acyl chain at the 3-position (i.e., are 3-O-deacylated) due to an alkaline hydrolysis processing step. *, denotes location of an unsaturated acyl group that represents the sole difference between ML5B and ML5C. Note that the 2′-position secondary acyl chains are slightly shorter, C12, than the other C14 chains and that the 2-position secondary acyl chain is slightly longer, C16. (B) Structures of synthetic Lipid A used in this study. PHAD (phosphorylated hexa-acyl disaccharide) is a synthetic 4′-monophosphorylated lipid A with six C14 acyl chains in the E. coli configuration (2:2:1:1); 3D-6A-PHAD is a synthetic 3-O-desacylated variant in the same configuration as ML6 (2:2:0:2) but with uniform acyl chain lengths. Lipid A, synthesized with six acyl chains and 1-, 4′-phosphates, is highly potent agonist of both mouse and human TLR4; Lipid IVa, synthesized with four primary acyl chains and 1-, 4′-phosphates, is an agonist of mouse TLR4 but a potent antagonist of human TLR4.
