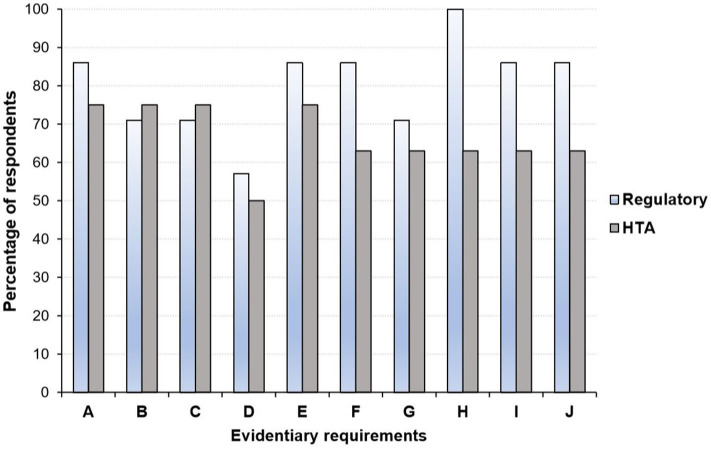
Figure 3.
Perspective of health technology assessment (HTA) assessors and regulators regarding areas where alignment in evidentiary requirement could occur*. (A) Acceptable primary end point. (B) Inclusion of active comparator arm in the trial. (C) Use of patient reported outcomes. (D) Use of health-related quality of life measures. (E) Choice and use of surrogate measures. (F) Criteria considered in choice of comparator: therapeutic. (G) Use of subgroup analyses. (H) Inclusion and choice of secondary efficacy parameters. (I) Definition of unmet medical need. (J) Use of biomarkers to monitor patient outcomes. HTA, health technology assessment. *Graph produced by author using data from Wang et al. (25) in which a questionnaire-based survey was conducted among regulators (n = 7) and HTA agencies (n = 8) between August and September 2016.