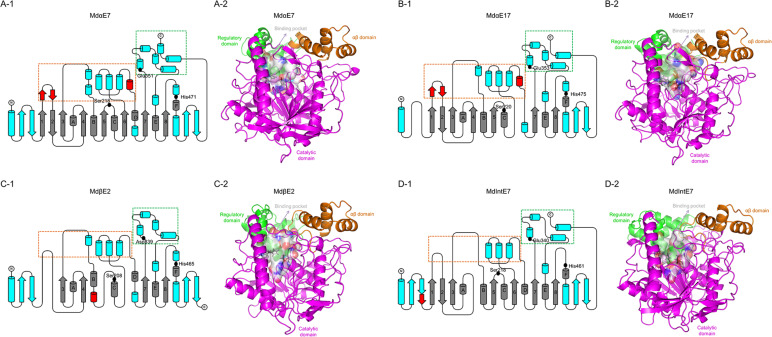
FIGURE 3.
The structures of carboxylesterases in M. domestica. (A-1), (B-1), (C-1), and (D-1) Topology representations of secondary structures of MdαE7, MdαE17, MdβE2, and MdIntE7 displaying the conserved α/β hydrolase fold (labeled as gray), conserved motifs among four carboxylesterase proteins (labeled as blue) and unique motifs belong to certain carboxylesterase structure (labeled as red). Two subdomains made up of bundles of α-helices (framed by orange and green box) formed the substrate binding cavity. Three conserved amino acids (Serine, Histidine, and Glutamine/Aspartic acid) consisted of a catalytic triad were also indicated as black dots. (A-2), (B-2), (C-2), and (D-2) Cartoon representations of structures of MdαE7, MdαE17, MdβE2, and MdIntE7 highlighting the regulatory domain (green area), αβ domain (orange area), catalytic domain (magenta area) and binding pocket (white surface area). The conserved catalytic residues were also labeled as black sticks.