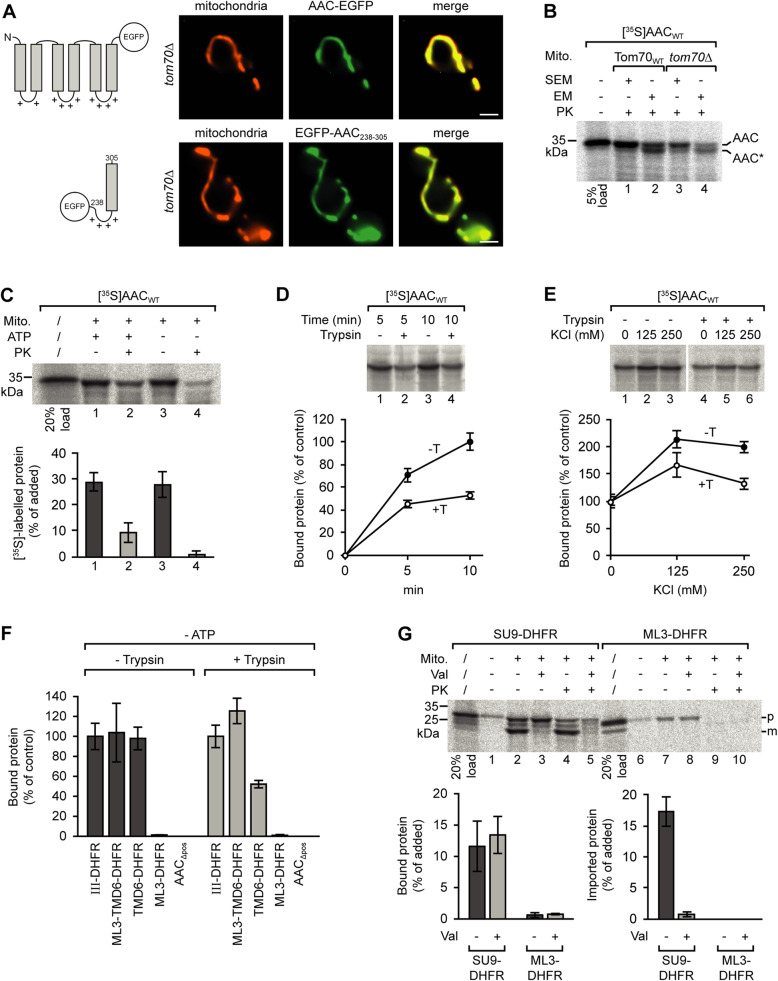
Fig. 2.
Tom70-independent targeting of AAC to yeast mitochondria. a Fluorescence microscopy of cells of a yeast tom70Δ strain expressing EGFP-labeled AAC (upper panel) or the EGFP-labeled segment containing the matrix loop of AAC module III and helix 6 (lower panel); scale bars 2 μm. b Assembly of the AAC in the mitochondrial inner membrane in WT and tom70Δ yeast mitochondria. Isolated mitochondria (30 μg) were incubated with reticulocyte lysate containing [35S]-labeled AAC at 25 °C for 10 min. The mitochondria were reisolated and resuspended in isotonic SEM or hypotonic EM buffer for opening of the mitochondrial outer membrane. To determine the amount of imported protein, the samples were incubated in the presence of 75 μg/ml proteinase K on ice for 10 min and the reaction was stopped by addition of 4 mM PMSF. The mitochondria were reisolated and subjected to SDS-PAGE. c Binding of [35S]-labeled AAC to yeast mitochondria. Isolated mitochondria and reticulocyte lysate containing [35S]-labeled AAC were separately depleted of ATP by incubation with apyrase. The mitochondria were subsequently incubated with the reticulocyte lysate (− ATP, samples 2 and 4). In parallel, untreated mitochondria were incubated with [35S]-labeled AAC in presence of 2 mM ATP (+ ATP, samples 1 and 2). The mitochondria of samples 3 and 4 were then treated with proteinase K. The mitochondria were reisolated, and the relative amounts of radiolabeled AAC were determined by SDS-PAGE and subsequent analysis using the phosphorimager. Standard deviations (SD), n = 4. PK, proteinase K. d Binding of [35S]-labeled AAC to trypsin-pretreated yeast mitochondria. The trypsin-pretreated mitochondria and reticulocyte lysate containing [35S]-labeled AAC were separately incubated with apyrase and subsequently mixed and incubated for 5 or 10 min at 25 °C. Untreated mitochondria (− Trypsin) were used in parallel. The mitochondria were reisolated to determine the relative amounts of bound AAC. The average amount of AAC bound to untreated mitochondria within 10 min was set to 100% (control). SD, n ≥ 6. e Binding of AAC to mitochondria in the presence of increasing concentrations of KCl. The average amounts of mitochondria-associated AAC obtained in the absence of KCl in the import buffer were set to 100% (control); SD, n = 3. f Binding of different [35S]-labeled polypeptides to trypsin-pretreated vs. untreated yeast mitochondria. The assays were carried out as in e using a buffer containing 80 mM KCl. The ATP-depleted reticulocyte lysates contained different segments of the AAC fused to dihydrofolate reductase (DHFR). ML3, matrix loop of AAC module III, residues 238–277; TMD6, transmembrane domain (AAC helix 6); AACΔpos, AAC with all positively charged residues of the matrix loops exchanged against glycine; SD, n = 3. g Incubation of isolated mitochondria with radiolabeled hybrid proteins containing the presequence (residues 1–69) of subunit 9 of N. crassa ATP synthase and DHFR (Su9-DHFR), or the matrix loop of AAC module III and DHFR (ML3-DHFR). SD, n = 3. Val, valinomycin; p, precursor protein; m, mature protein